1. Introduction
Community-acquired pneumonia (CAP) is one of the most prevalent diseases that remains a severe illness and has a high rate of morbidity and mortality despite antibiotics treatment.[1] The mortality of pneumonia has increased to the point where it became the 3rd most common cause of death in Korea in 2019 (death rate in Korea; 45.1 per 100,000), from the 9th cause in 1999.[2] The increasing medical expenses for CAP has become a significant economic burden on the society.[3] Recent studies have focused on treatment for CAP through early diagnosis and adequate empirical antibiotic therapy according to age, comorbid illness, and severity of illness.[4]
There have been a few studies that determined the effect of corticosteroids as an adjuvant therapy for CAP. A previous study reported that administration of systemic corticosteroids alleviates systemic and pulmonary inflammatory responses in patients with severe pneumonia alongside adequate antibiotic management[5] This implies that anti-inflammatory effect of systemic corticosteroids may improve pneumonia severity and the patient’s outcome such as length of hospital stay.[6] A recent meta-analysis reported that systemic corticosteroid reduced mortality by approximately 3%, and length of hospital stay by 1 day[7], while other studies showed limited evidence which supported the efficacy of systemic corticosteroids in patient with CAP[8]. Some studies even reported increased risk of steroid-related side effects such as super infections and hyperglycemia.[9] Although CAP is a highly prevalent disease in children, there have been no studies determining the effect of systemic corticosteroids on CAP in pediatrics populations.
The aim of this comprehensive review is to investigate the efficacy of systemic corticosteroids administration in children with CAP whose signs and symptoms worsened despite appropriate antibiotic treatment. Our hypothesis is that systemic corticosteroids may reduce systemic inflammation and regulate pro-inflammatory, anti-inflammatory cytokines in children with CAP.
2. CAP definition
CAP is diagnosed when a chest radiograph shows a new pulmonary infiltrate with the presence of acute pneumonia associated with at least one of the following criteria; temperature higher than 38°C or lower than 35°C, new cough with or without sputum production, dyspnea, pleuritic chest pain, or altered breath sound on auscultation[10].
Globally, lower respiratory infections are included in the International Classification of Diseases (ICD)-9 codes (079.6, 466 to 469, 470.0, 480 to 482.8, 483.0 to 483.9, 484.1 to 484.2, 484.6 to 484.7, and 487 to 489) and ICD-10 codes (A48.1, A70, B97.4 to B97.6, J09 to J15.8, J16 to J16.9, J20 to J21.9, J91.0, P23.0 to P23.4, and U04 to U04.9).[11]
3. Global burden of lower respiratory infections
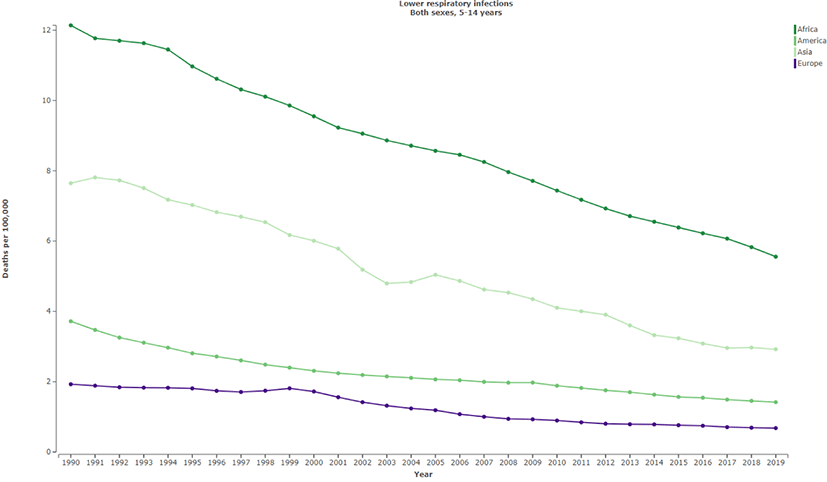
Based on the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 methodology from 1990 to 2019, we evaluated the incidence and mortality in children (5 to 14 years) throughout 204 countries and territories.[12, 13] We reported decreasing trends in incidence rates of lower respiratory infections in children (5 to 14 years) globally (Fig. 1). Furthermore, death rates due to lower respiratory infections appeared to decline between 1990 and 2019 at a global level (Fig. 2). There were 3.27 deaths (95% uncertainty interval [UI], 2.78 to 3.84) reported in 2019 and 7.22 deaths (95% UI 6.06 to 8.34) in 1990 due to lower respiratory infections.
4. Steroid therapy
Previous studies have reported a beneficial effect of systemic corticosteroids in adult patients with CAP. A recent study involving 304 adult patients with CAP, randomly allocated them into the placebo and dexamethasone groups and found a significant reduction in hospital mortality and length of hospital stay in patients with severe CAP who were given a bolus of 5 mg of dexamethasone intravenously for 4 days.[14] Furthermore, a retrospective and observational study determined that mortality decreased in patients with severe CAP who were given systemic corticosteroids (either methylprednisolone or prednisone) along with antibiotic treatment when compared with patients who did not receive systemic corticosteroids.[15] Recently, a randomized, double-blind, placebo-controlled trial assessing adult populations with CAP found that CAP patients treated with corticosteroid had reduced risk of failure of treatment.[16] Corresponding well with previous studies of adult patients with CAP, we found that systemic corticosteroids had a significant effect in reducing systemic inflammation as reflected by a reduced fever within 24 hours of systemic corticosteroids administration (Table 1).[17-19]
The systemic anti-inflammatory effects of corticosteroids on the immune system are complex. Possible mechanisms are as the following. First, corticosteroids may modulate secretion of cytokines that play a key role in the pathogenesis of pneumonia. Circulating level of the systemic pro-inflammatory cytokine (interleukin [IL]-1β, IL-6, and tumor necrosis factor [TNF]-α) and anti-inflammatory cytokine (IL-1 receptor antagonist and IL-10) are activated in patients with CAP.[20] The administration of corticosteroids increases anti-inflammatory activity and decreases pro-inflammatory activity in multidimensional ways which include its direct effect by binding of glucocorticoid receptors to glucocorticoid-responsive elements (i.e., the induction of annexin I and MAPK phosphatase 1), indirect effects through the interactions of glucocorticoid receptors with other transcription factors (i.e., NF-kB and activator protein 1) on gene expression, and glucocorticoid receptor-mediated effects on second-messenger cascades (i.e., the PI3K–Akt–eNOS pathway).[21] Therefore, treatment with low doses of corticosteroids interrupts pro-inflammatory cytokine transcription and facilitates anti-inflammatory cytokines transcription.[14, 15, 22, 23] Second, administration of systemic corticosteroids into patients with CAP improves gas-exchange function by relieving fever and modulating excessive aforementioned cytokine responses.[17-19] Third, although the study subjects included in the current study were not admitted to an intensive care unit, there are studies reporting that administering systemic corticosteroids may improve adrenal insufficiency often observed in a high proportion of patients with severe CAP admitted to the intensive care unit.[24] Taken together, corticosteroids may have beneficial effects on the immune response and therefore improve disease severity[14, 22] and decrease febrile duration[25] in the early phase of CAP.
The optimal choice of corticosteroids for adjuvant management in pediatric CAP is currently controversial due to the fact that the use of systemic corticosteroids for CAP in the pediatric population has not been as widely reported as other diseases, such as acute respiratory distress syndrome, bacterial meningitis, and septic shock. A previous case study looking at a 9-year-old girl with severe CAP reported that continuous hydrocortisone infusion in the patient with CAP was effective in mediating the severe inflammatory process.[26] Other studies have suggested that methylprednisolone and prednisolone may have a beneficial effect in reducing morbidity and mortality in antibiotic non-responsive Mycoplasma pneumoniae and some virus-induced pneumonia. [17-19] Our study evaluated the benefits of systemic corticosteroids, which has a longer biological half-life of 36-54 hours and slower reduction of biological response such as intracellular steroid effect and hypothalamic-pituitary-adrenal axis recovery when compared with other corticosteroids.[27, 28]
Despite apparent evidence that systemic corticosteroid has beneficial effects, it may also cause undesirable side effects. A recent study reported adverse effects of systemic steroid in severe pediatric CAP, such as corticosteroid-associated impairment of the host immune response, ultimately resulting in a longer length of hospital stay and higher hospital readmission rates, hypertension, and hyperglycemia.[29] Further research is needed to determine the detrimental effects of systemic corticosteroids in children with CAP.
5. Conclusion
Adequate empirical antibiotic therapy with intravenous administration of systemic corticosteroids as an adjuvant therapy improved clinical signs and symptoms in hospitalized children with CAP without significant side effects. This comprehensive review suggests that systemic corticosteroids treatment may be beneficial for reducing morbidity in hospitalized children with CAP when used with antibiotics.







