1. Clinical specifications and global infection status of the mpox
The mpox (monkeypox) is a zoonotic acute rash infectious disease caused by the infection of Orthopoxvirus.[1] It was first discovered in a laboratory breeding monkeys in 1958 and was given the name mpox virus (MPXV) because it was similar to the smallpox but caused slightly less severe symptoms.[2] Later in the 1970s, it was reported that viruses of the smallpox was discovered only in animals such as non-human primates and rodents in Congo.[3] However, even when the smallpox was eradicated in the 1980s, the mpox continued to occur in many countries across Midwest Africa, and due to the eradication of smallpox and prevention of smallpox through vaccines, the mpox has been eliminated from the most serious Orthopoxvirus transmission through public health. Before it became prevalent around the world in 2022, it was only an endemic disease mainly found in rural rainforest areas in Central and Western Africa.[4]
2. Transmission of MPXV to human
The Orthopoxvirus is composed of two types of clades: the West African clade and the Congo basic clade, also called the Central African clade. The clade currently breaking out is the West African clade. Although it has a low fatality rate of 1%, due to the fact that the Central African clade has a high risk of transmission and a fatality rate of 10%, more thorough prevention is needed.[5]
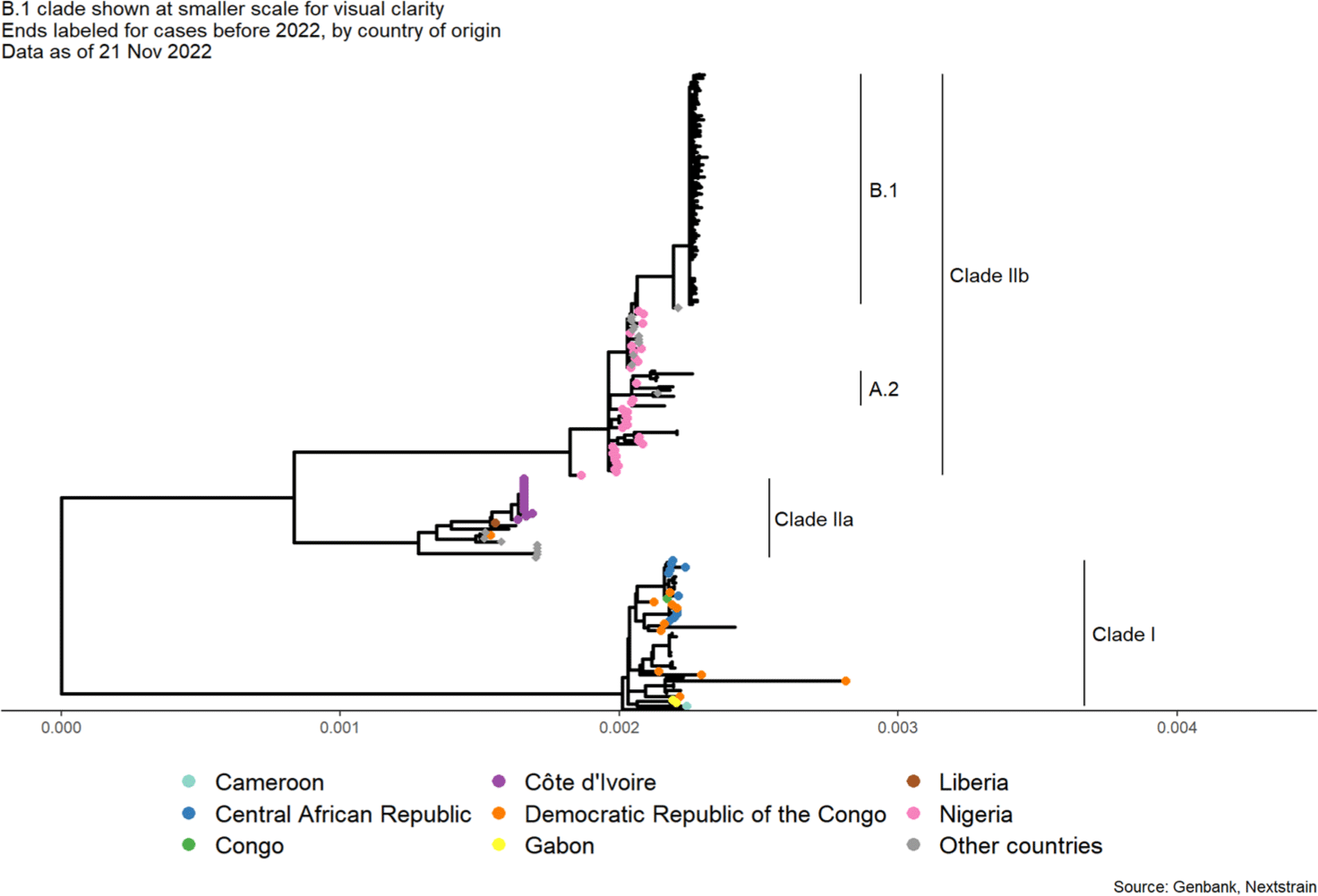
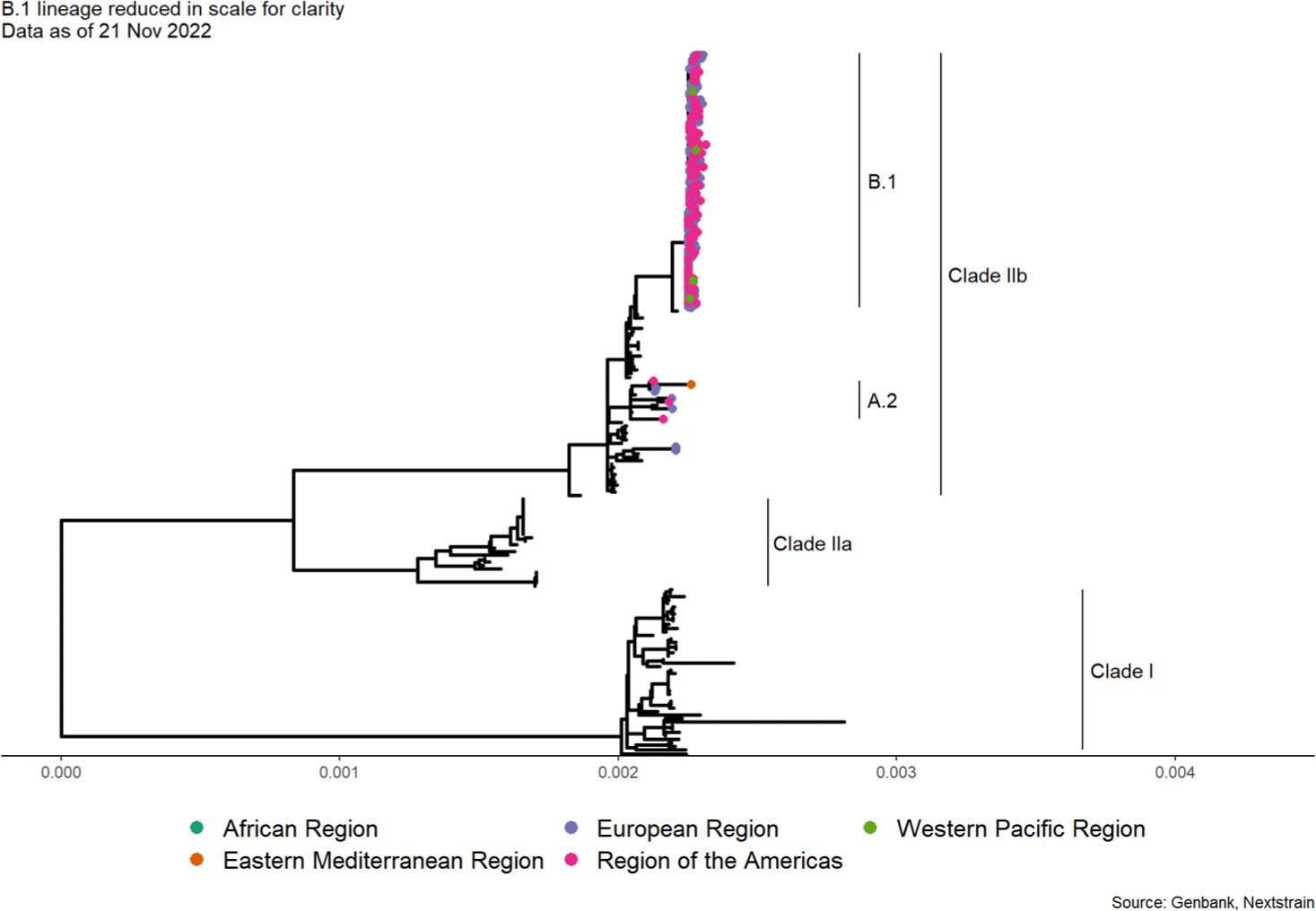
Since the mpox is a zoonotic infectious disease, infection from animals which were previously infected by other animals is dominant, but due to the recent surge of human-to-human transmission cases, special attention is required.[6] According to Andrew Lambaut et al. research team at University of Edinburgh, the MPXV, which was prevalent in 2022, is very similar to the MPXV which occurred on a small scale in Singapore, Israel, Nigeria, and the United Kingdom in 2017 (Fig. 1 and Fig. 2).[7] When comparing the sequences of the two viruses in different periods, 47 base-sequence were changed, with 42 of the 47 mutations where guanine which converted to adenine or cytosine was replaced with thymine.[6] All of these mutations correspond to the human enzyme protein apolipoprotein B mRNA editing enzyme catalytic polypeptide 3, which prevents various kinds of viruses.[8] In other words, since penetration of Orthopoxvirus into human body was first observed in 2017, it has developed a new mutation by fighting the immune system in the process of replicating itself.[9] This indicates that although the Orthopoxvirus is a DNA virus has a low success rate and usually mutates once a year, a much higher number of mutations have happened than expected.[9] In addition, human-to-human transmission is known have the longest documented chain of transmission which ranged from six to nine generations with the last person infected in this chain being at most nine links away from the first infected person.[10] This shows a decline in immunity in all communities due to the suspension of smallpox vaccination.
Pathways of human-to-human infection of the Orthopoxvirus include direct contact with fluid, skin, and mucosal lesions to symptomatic patients, mediated contact of objects or fabrics used by infected people, and vertical infection from the mother to fetus through the placenta.[5] Although droplet infection by respiratory secretions is possible, infection is less likely to occur compared to other respiratory infectious diseases because it requires long-term face-to-face contact in general.[10] The possibility of transmission through droplet infection is low unless in close contact with active patients, such as medical workers or household members of the patient.[11] Intimate physical contact is also well known as a risk factor for mpox transmission, but further evidence is needed to prove that mpox can be specifically transmitted through the sexual transmission route. In addition, although it has been confirmed that air transmission through micro aerosols containing Orthopoxvirus between animals is possible, a better understanding is needed to confirm whether the air transmission between humans is possible.[5] Currently the method of transmission of Orthopoxvirus from animals to humans or the specific animal host reservoir of mpox remains unknown.
3. The tendency of patients with mpox
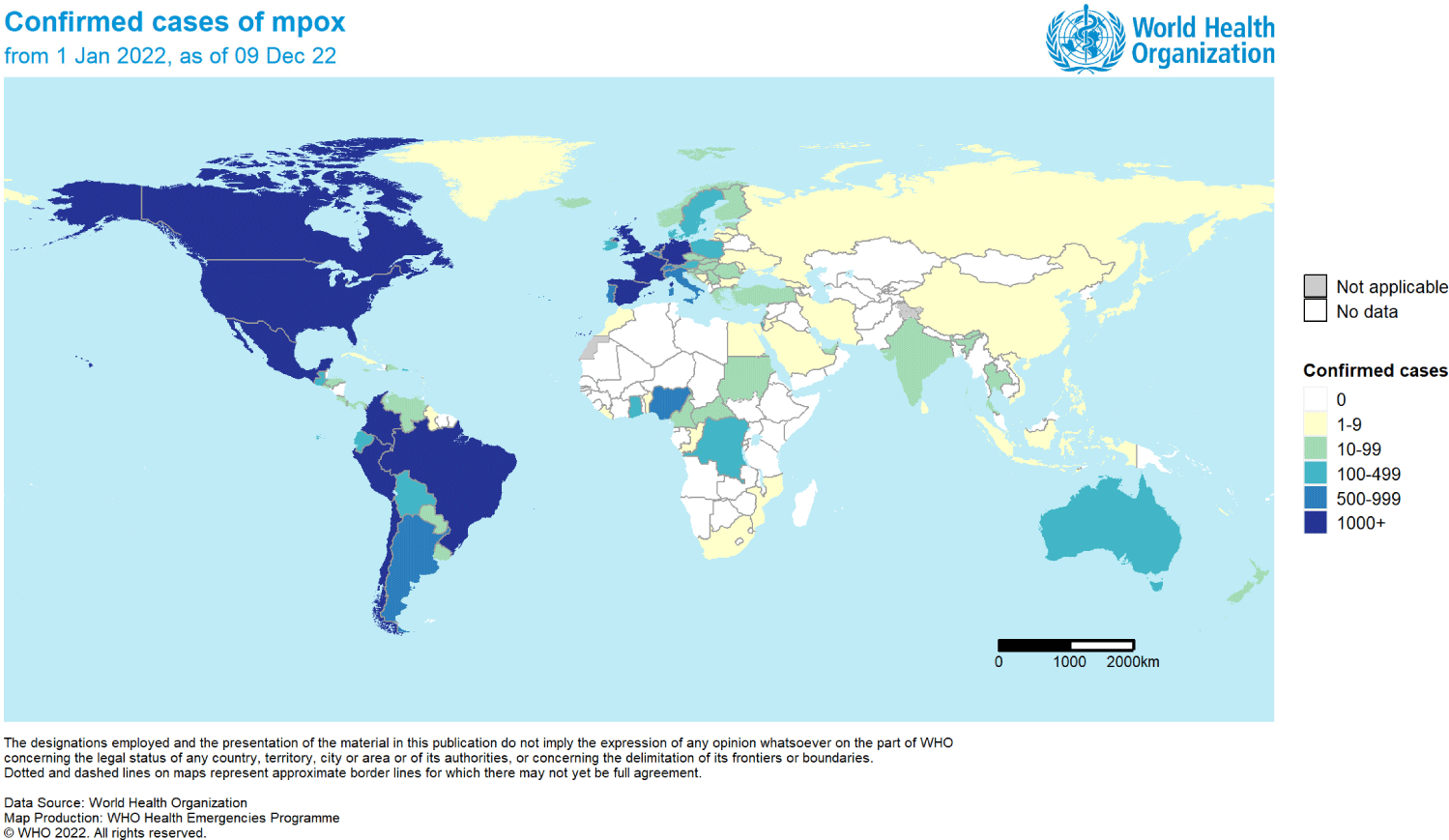
The above figure shows confirmed cases of mpox from January 1 to December 9, 2022. The white-colored-part represents 0 cases, lemon-colored-part 1 to 9 cases, mint-colored-part 10 to 99, aquamarine-colored-part 100 to 499, blue-colored-part 500 to 999, and navy-colored-part over 1,000 confirmed cases (Fig. 3). More than 1,000 cases have been identified in North America and certain Western European countries as well as in Central and Western Africa, which allows analyzing and confirming the transmission path and infection trend of the mpox disease which started in African regions and eventually extended to the Americas and European regions through long-term DNA mutations.
According to reports from the World Health Organization (WHO) regarding cases in 110 countries, 82,594 cases were confirmed and 55 deaths were reported as of 9 December 2022 (Table 1). The most confirmed cases were identified in the Region of the Americas with a total of 55,695, followed by 25,581 cases from European Regions. Notably, even though Midwest Africa was the source of the disease, there were only 982 confirmed cases in the African Region. 226 cases were confirmed in the Western Pacific Region, 78 cases in the Eastern Mediterranean Region, and 32 cases in the South-East Asia Region, which showed relatively low numbers of confirmed cases.
According to the case profiles surveyed by the WHO, men who had sex with the same sex accounted for the highest proportion of confirmed cases of 86.1%, while sexual transmission also showed the second highest proportion at 70.5%. This was followed by 51.5% of people living everyday lives with human immunodeficiency virus (HIV), 15.6% through travel, 7.1% while hospitalized, 4.8% health workers, and 0.2% as intensive care unit admission as causative variables of mpox. Naturally as men who had sex with the same sex accounted for the highest proportion, 96.8% of all patients were male, and the average age was found to be 34 years old. It is possible that bisexual men, which made up the 5.3% of men who have sex with men group, could have transmitted Orthopoxvirus to women, who made up 3.2% of the total and is seen as the main reason of transmission within household (43%). The statistics show that 42% of women are transmitted due to sexual encounters, but the fact that 87% of them were heterosexual is the main difference from cases of men. As can be seen from Table 2, most of the variables acting as factors of mpox are related to sexual contact, especially men and HIV. The fact that the number of confirmed cases increases even though patients didn't have any travel history to endemic region can be seen as a threat to public health.
4. Symptoms associated with mpox
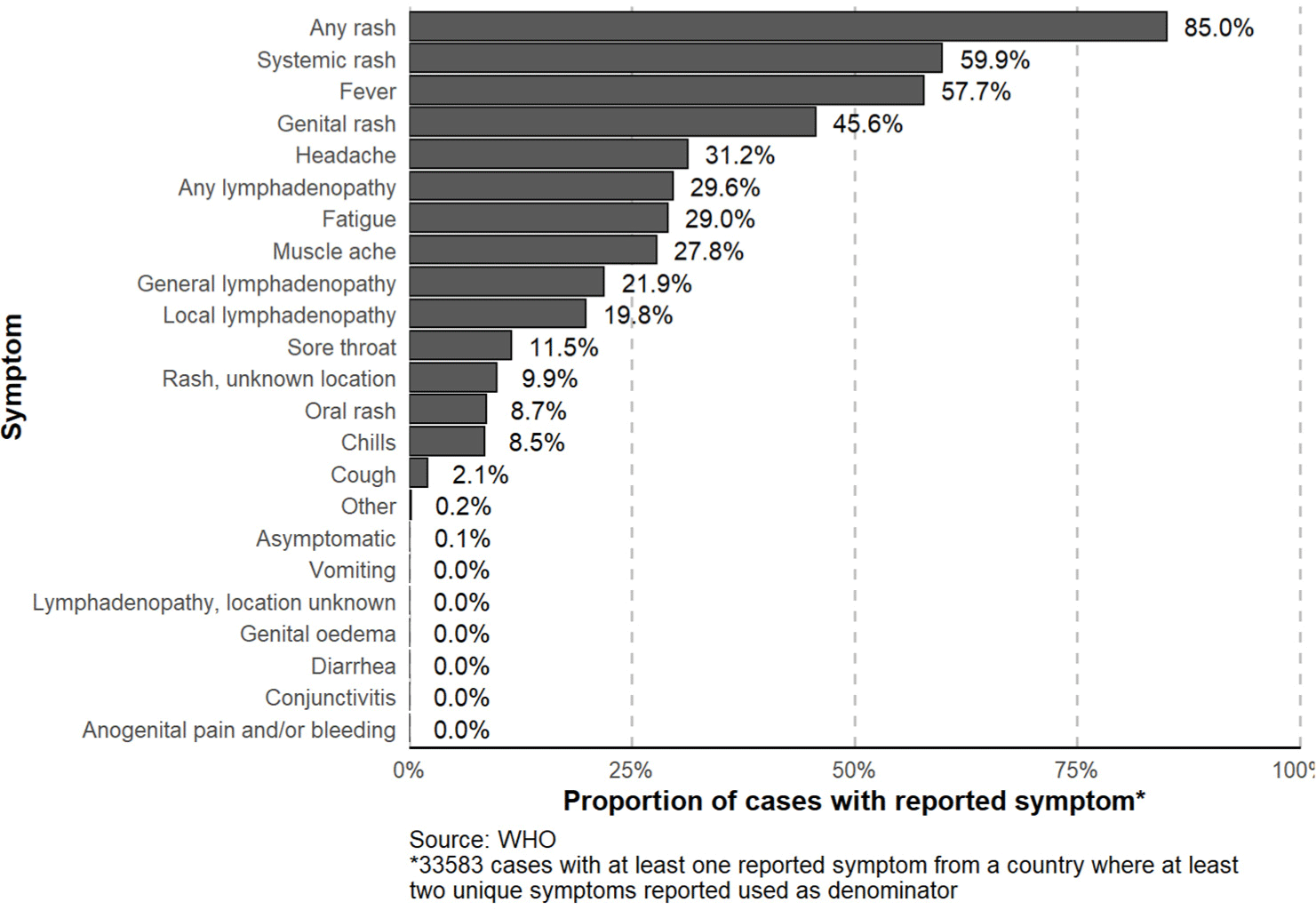
The clinical symptoms of mpox such as rashes and lesions are difficult to distinguish from the smallpox . Most cases of the outbreak in current showed mild disease symptoms which had little influence on the fatality rate, but the mpox is still capable of causing serious diseases in certain groups with weak immunity, including young children and pregnant women.[12] The most common symptom among the reported symptoms was rash (all kinds), reporting 85% of the cases which included one or more rash symptoms, including systemic rash, genital rash, and oral rash. As of December 9, 2022, 28,551 patients, accounting for 85% of all patients, reported at least one type of rash, of which systematic rash was 59.9%, genital rash was 45.6%, rash in unknown location was 9.9% and oral rash was 8.7%. 19,390 patients showing fever accounted for 57.7% of the total, and patients showing headache and fatigue accounted for 31.2% and 29.0%, respectively. There are also many patients showing symptoms of lymphadenopathy, including general lymphadenopathy and local lymphadenopathy. 9,957 patients reported cases of lymphadenopathy which accounted for 29.6% of the total, of which 21.9% were general lymphadenopathy patients and 19.8% were local lymphadenopathy patients. In addition, various symptoms such as sour through (11.5%), chills (8.5%), coarse (2.1%), and asymptomatic (0.1%) were reported (Fig. 4).
5. Diagnosis
When mpox is diagnosed, the diagnosis must clinically differentiate mpox from other rash diseases such as chickenpox, measles, bacterial skin infections, scabs, syphilis, and drug-related allergies. Here, lymphadenopathy can be an important clinical division method that distinguishes mpox from smallpox and chickenpox. If there is a possibility of mpox infection, health workers collect necessary samples such as blister lesions, exudates, or skin lesions through oropharyngeal or nasopharyngeal swab and conduct the diagnosis in a laboratory. The samples collected and the type of laboratory test performed are the elements which have influence on the diagnosis.
The Polymerase Chain Reaction (PCR) is a normally preferred method for the diagnosis of mpox due to its high accuracy and sensitivity.[13] The lesion sample is stored cold in a dry sterilized tube for diagnosis. In the case of PCR blood tests, PCR-based diagnosis is generally inconclusive because the duration of viremia is shorter than when the sample was collected after the onset of symptoms and should not be collected regularly from the patient.
Serologic test utilizes Orthopoxvirus-specific immunoglobulin detection, in which paired acute and convalescent sera is required. Immunoglobulin M detection is performed within 5 days and immunoglobulin G detection is performed after 8 days of mpox infection.[14] However, since Orthopoxvirus is serologically cross-reactive, the mpox cannot be specifically identified through antigen-antibody detection methods. Therefore, serology and antigen detection methods are not recommended for diagnosis when the resources are limited. There is a possibility of misdiagnosis due to vaccinia-based vaccines such as vaccines inoculated before treating a smallpox.
In addition, biopsy, sequencing, and diagnosis using an electron microscope can be used for mpox diagnosis. In interpreting the diagnosis results, it is important to provide enough patient information such as the start date of fever and rash, the sample collection date, the stage of symptoms, and age.
6. Treatment
Methods for treating mpox include supportive care, medication such as anti-virals, tecovirimat, brincidofovir and cdofovir, and vaccinia immune globulin.[15] Since most patients with mpox are treated individually without special medication, appropriate supportive care is required depending on the symptoms. For example, patients with gastrointestinal symptoms, such as vomiting and diarrhea, need desperate hydration to minimize water loss.[16] Likewise, appropriate supplementation can alleviate symptoms, manage complications, and prevent long-term aftereffects. Some antiviral supplementation may be effective in treating mpox, but these drugs have been approved for smallpox management based on animal models. Although dose studies have been performed on these drugs according to the human body, the efficacy of the drug cannot be completely trusted.[16]
Tecovirimat, the first smallpox treatment, is also an antiviral drug.[16] It inhibits the spread of the virus within the infected host by inhibiting the viral envelope protein VP37, such as hindering the maturity of the Tecovirimat virus and blocking the final step of releasing the virus from the cell.[16] In a related study, it was used in conjunction with vaccinia immune globulin in patients with complications of smallpox vaccines such as eczema and progressive vaccines.[17] Tecovirimat has been licensed by the European Medicines Agency as a 2022 mpox treatment through clinical research on animals and humans but is not yet widely available to the public. Additional clinical data collection and monitoring is essential when used.[17]
Brincidofovir is a smallpox treatment approved in the United States in 2021, and is an analog that improves kidney toxicity problems in cidofovir.[18] These drugs work by inhibiting viral DNA polymerase, which has been shown to be effective in treating MPXV infections in animal models. Although clinical data on the efficacy of cidofovir in human mpox treatment are insufficient, it is likely to act as a treatment because positive in vitro activity and efficacy have been reported for the treatment of fatal Orthophoxvirus in animals.[18] Since brincidofovir can increase serum amino group transferase and serum bilirubin, it can be recommended that liver function tests be performed before and during treatment, and normal saline and probenide treatment in the vein be combined with cidofovir.[18]
Vaccinia immune globulin is a highly immunoglobulin licensed by the U.S. Food and Drug Administration for the treatment of certain complications such as eczema vaccinatum, progressive vaccinia, severe generalized vaccinia, and vaccinia infections of the vaccinia vaccine.[19] Since vaccinia immune globulin has not been tested on mpox and smallpox in humans, there is insufficient data to prove its effectiveness. However, as vaccination with vacinia virus vaccine is prohibited in patients with severe immune deficiencies in T-cell function, despite the deficiency of data, vaccinia immune globulin can be seen as a potential alternative treatment.
7. Prevention
While the prevention of mpox spreading in endemic areas is very challenging mission and is generally tried to be accomplished through methods avoiding contact with infected animals, the recent prevention of human-to-human transmission of mpox is focuses on restrictions of contact with infected patients. If a suspected patient visits the hospital, the most recommended step is the patient being placed in a negative pressure isolation room or in a private room immediately. In addition, as the smallpox vaccine is estimated to provide 85% cross-protection against MPXV infection, it is helpful for people who are constantly exposed to Orthopoxvirus, such as healthcare workers or patients with mpox, be vaccinated against smallpox.[12]
Collecting samples of viruses from patients is subject to standard infection control prevention measures and the samples are advised to be handled by specialized personnel in appropriately equipped laboratories.[12, 13] In addition, special care should be taken to prevent the sample from spreading during the movement of the sample.
When caring for patients, it is advantageous to prevent transmission by selecting people who have previously been vaccinated against smallpox.[20] Raising awareness of the risk factors of the disease and educating the public is one of mpox's main prevention strategies of the World Health Organization. Large-scale health education campaigns highlight the risk of the disease and inform host animal species to prevent infection through ingestion or contact. In addition, further infections among healthcare workers in hospitals can be prevented by informing them of the importance of using sanitary medical equipment such as gloves, protective clothing, and surgical masks.[21]
8. Conclusion
Zoonosis, such as the mpox, is a kind of spillover which refers to a phenomenon when a virus that lived in a particular species crosses the interspecies barrier and spreads to other hosts due to various factors.[22] This happens because environmental changes which make spillovers easily occur, and considering that all infectious diseases except the cholera began by spreading from animals to humans in the last century, it can be recognized as a serious threat to the public. Fatal epidemics such as the Middle East respiratory syndrome coronavirus, Ebola, Novel swine-origin influenza, severe acute respiratory syndrome, Zika virus infection, and Nipa virus infection, including mpox and severe acute respiratory syndrome coronavirus 2, which were relatively recently prevalent worldwide, and the mentioned diseases were infected to humans due to contact with host animals. Also, HIV, one of the worst diseases in human history, was also zoonosis.[22] Due to barriers, interspecies transmission usually does not occur easily but when the virus combines with the receptor through mutations, it causes constant human infection.
Among various reasons, many scientists are focusing on the climate crisis. Rising temperatures change the condition of the land which forces farmers to reclaim it and thus deprives the existing animals of their habitats.[23] Animals that have lost their habitats enter human habitations which leads to risk of increasing human-to-animal contact, eventually resulting into the outbreak of new zoonosis. In addition, when their predators are endangered due to the climate crisis, the number of rodents increases rapidly, enhancing the possibility of transmitting diseases to humans.[23] Similarly, industrialization and the movement of people around the world increases close human-to-animal contact.[24] Previous studies predicted that the climate crisis will increase the number of virus transmission between animals by more than 15,000 over the next 50 years, and even if global warming controlled to occur within 2 degrees Celsius, the risk of infection increases as 3,139 species of habitats move by 2070.[25] To prevent the recurrence of existing diseases such as mpox and the spread of new diseases, future studies to understand the causes and mechanisms of spillover and to preserve biodiversity on a global level is essential.