1. Introduction
Vaccines, like infections, activate the immune system which could eventually trigger the development of an autoimmune disorder like ITP (immune thrombocytopenic purpura) or TTP (thrombotic thrombocytopenic purpura) or Guillain–Barre syndrome.[1] In thrombosis, viral proteins and free DNA in the vaccine bind to platelet factor 4 (PF4) to generate a neo-antigen that subsequently leads to the development of antibodies promoting platelets activation and clotting.[1] Platelets function as immune cells in conjunction with white blood cells, targeting invading pathogens and inducing immune reactions. Intercellular communications among these immune cells are partly mediated by platelet polyphosphate (polyP), which was originally recognised as a thrombotic and haemostatic biomolecule.[2] Platelets are activated by Immunoglobulin G (IgG) through FcγRIIA (also known as CD32a).[2] PF4-polyphosphates-Ig immune complexes bind to FcγRIIA on the surface of platelets and thus cross-link these receptors, inducing platelet activation and perpetuating over time a platelet activation/consumption and prothrombotic state even without the presence of heparin.[3] Polyphosphates contained in the dense granules of platelets are able to induce autoactivation of Factor XII and trigger the contact phase-dependent coagulation cascade.[4] Thromboembolic events are probably caused by impaired binding of clotting factor X to the viral capsid. The unprotected capsid then stimulates an immune response leading to platelet activation, increased thrombogenicity, and formation of an antibody complex with PF4. Impaired factor X binding may be due to an undiagnosed mutation in affected individuals.[5]
2. Potential molecular mechanisms resulting in VITT
After secretion, PF4 may bind other ligands with higher affinity, such as endothelial-derived perlecan heparan sulphate side chains.[5] It is thought that the polyanions allow conformational changes in PF4 exposing antigenic determinants for anti-PF4 IgG antibodies.[6] It is possible that antibodies are induced by continued viral infection, and that the adenoviral vector itself activates platelets and provides an early trigger for PF4 secretion. These strong antibodies, if released in sufficient titers, are able to aggregate PF4 in a ligand-independent manner.[7] After vaccination and consequent possible viraemia, ChAdOx1 nCov-19 particles, can directly reach different cell types, including platelets and endothelial cells, causing severe thrombocytopenia.[7] Greinacher et al. observed a strong activation of platelets by ChAdOx1 nCov-19.[8] Positively charged structures on the AdV surface that bind negatively charged glycosaminoglycans, might elicit antibodies cross-reacting with PF4, which would explain that both PF4 and the AdV components of the vaccine enhance platelet activation.[8] It remains to be determined whether the antibodies found are auto-antibodies against PF4 induced by the strong inflammatory stimulus of vaccination or vaccine-induced antibodies that cross-react with PF4 and platelets.[8]
Pang and collaborators proposed five potential anionic substances of the ChAdOx1-S vaccine that can combine with PF4 and trigger VITT, including (1) the proteins on the surface of adenovirus, e.g., negative charged glycoprotein; (2) the adjuvant components of the vaccine, e.g., Tween 80; (3) the DNA of adenovirus; (4) the S protein antigen expressed by the vaccine; and (5) the negatively charged impurity proteins expressed by the vaccine, e.g., adenovirus skeleton proteins. After analyzing each case, they considered that the most likely trigger was a negatively charged impurity proteins expressed by the vaccine. Accordingly, the susceptible individuals of VITT after ChAdOx1-S vaccination may be those expressing negatively charged impurity proteins that could be detected in the sera of VITT patients by quantitative proteomics, or by isolating and purifying the cations of the impurity proteins and testing their PF4 binding capacity.[9]
Recently, Kanack and collaborators revealed the difference between the development of platelet-activating anti-PF4 antibodies and the thrombotic thrombocytopenia syndrome seen after ChAdOx1 nCoV-19 and Ad26.COV2.S vaccination and Heparin induced thrombocytopenia (HIT) [9], indicating that clonally restricted anti-PF4 antibodies mediate vaccine-induced immune thrombotic thrombocytopenia (VITT) while polyclonal anti-PF4 antibodies mediate HIT. In VITT, the strong immune response may result in the activation of a single or few pre-existing anti-PF4 reactive clones, and development of clonally restricted anti-PF4 antibodies with a similar pathophysiology to spontaneous HIT.[9]
3. Immunologic responses
Anti-PF4/polyanion IgG-mediated thrombus formation in patients with VITT is accompanied by a massive innate immune activation, and particularly the fulminant activation of neutrophils including NETosis (Fig. 1). Intravascular administration of AdV-S induces both innate and adaptive immune responses characterized by increased levels of cytokines and chemokines. Thus, intravascular application of AdV-based vaccines induced inflammatory responses, as well as interaction with platelets, endothelial cells, and the coagulation cascade.[10] Thus, AdV-S vaccine such as ChAdOx1 nCoV-19 may contribute to thrombocyte activation.[9] A post-mortem study of VITT showed large venous vessels involvement in thrombotic occlusions in the microcirculation of multiple organs as well as increased inflammatory infiltrates, suggesting a progression of an inflammatory process that culminates in microvascular injury of multiple organs by iatrogenic activation of the innate immune system along with the complement system.[11] ChAdOx1 leads to an inflammatory response with increased levels of interleukin (IL)-6.[12] In hospitalised patients with COVID-19, prothrombotic antibodies that activate neutrophils, platelets, and endothelium have been identified.[12]
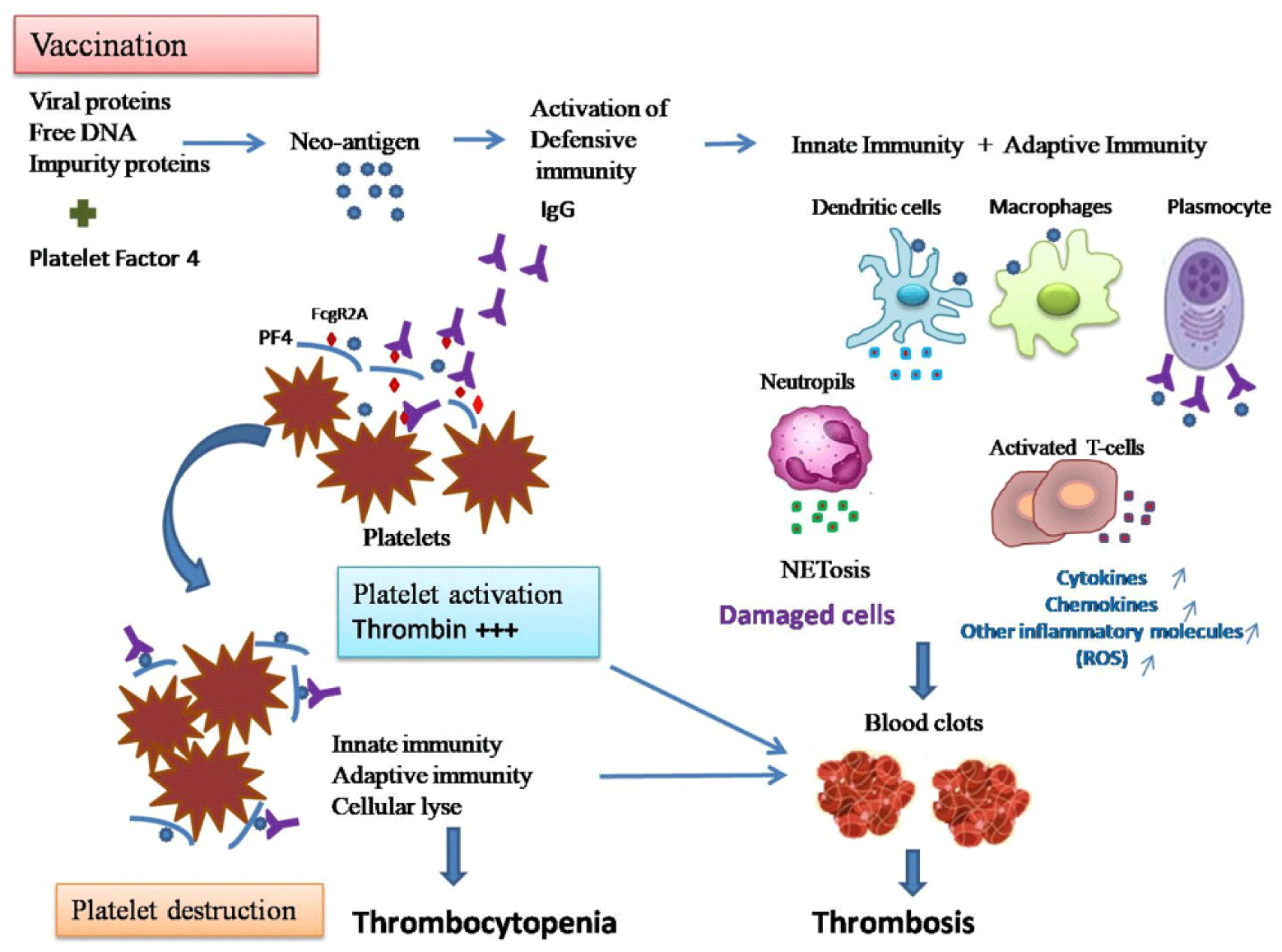
Immune assays and immune cell phenotyping by flow cytometry analyses and immunoprecipitation with anti-PF4 antibody in plasma samples followed by mass spectrometry revealed circulating inflammatory markers.[13] Precipitated immune complexes indicated that multiple innate immune pathways trigger platelet and leucocyte activation. In plasma samples, levels of innate immune response cytokines and markers of systemic inflammation increased, alongside extensive degranulation of neutrophils, formation of neutrophil extracellular traps (NETs), IgG deposits, increased levels of circulating H3Cit, dsDNA, and myeloperoxidase (MPO)–DNA complex. Indirect signs of NET formation in peripheral blood of patients are found including H3Cit, dsDNA, and MPO–DNA complex as opposed to healthy controls and vaccinated healthcare workers without signs of thrombus formation, and tissue and endothelial damage.[13] The study highlighted that the role of innate immune responses in VITT includes an unusual fulminant focal neutrophil activation in cell-rich thrombi as well as systemic activation of leucocytes and circulating cytokines, free nucleic acids, and acute phase reactants. Increased levels of the alarmins S100A8 and S100A9 were observed both in circulation and in the sinus thrombus.[13] NETs, consisting of neutrophil-derived chromatin associated with pro-coagulant proteins and antimicrobial proteins, such as MPO or neutrophil elastase are present abundantly in thrombotic events. COVID-19 is characterised by a high prevalence of thrombotic complications.[13]
PF4 can bind and aggregate DNA (as a polyanion) and amplify toll-like receptor 9 (TLR9) signalling by organising fragmented DNA into liquid-crystalline integrated circuis with inter-DNA spacings optimal for TLR9 amplification. This suggests a positive feedback loop that would be further stabilised by anti-PF4 IgG. Thus, it is likely that the FccRIIA, complement C5a receptor 1, and TLR9 signalling converged in a massive activation of neutrophils in the patients.[13] In VITT, this might be caused by platelets’ direct activation by the ChAdOx1 nCoV-19 adenoviral vector vaccine degranulation and PF4/glycosaminoglycan secretion, exchange of glycosaminoglycan with an unknown polyanion, generation of IgG against PF4/polyanion. The chemokine PF4/polyanion activates leucocytes via the CXCR3-B splice variant of CXCR3 and CCR1.[14] Following the formation of PF4—adenovirus complexes, T cell, especially within the CD4+ subset, responses against prior adenovirus infections may provide help to B cells in the generation of anti-PF4 response.[15]
The strong proinflammatory T cell responses induced by vaccination could also induce the anti-PF4 antibody response in VITT, as IL-10–producing regulatory T cells have been demonstrated to suppress PF4/heparin-specific antibody responses during HIT in mice.[16] It has been shown that SARS-CoV-2infections itself can also induce a diverse array of functional auto-antibodies in the host.[16] Future studies should profile autoantibody production resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and the proposed autoantibodies resulting from vaccination to establish any possible links between the presence of autoantibodies and thromboembolic events.
4. Therapeutic approach based on immunology
To address proper treatment, it is important to diagnose VITT and to exclude other potential causative factors resembling the disease. Based on its resemblance with HIT, several therapeutics have been proposed to manage VITT, but results are uncertain and research is still ongoing. PCR testing has shown negative SARS-CoV-2 infection in many but not all patients with VITT. Therefore, whether VITT is an atypical form of COVID-19 requires further studies.[17] Management of VITT generally includes heparin avoidance, use of alternative (non-heparin) anticoagulants, and intravenous immunoglobulin. The antibodies involved in thrombocytopenia after AdV-S vaccination occurred without any prior heparin therapy, and their effect on platelet activation was rather blocked by heparin.[17] In VITT, heparin should be avoided as it is difficult to exclude cross-reactivity between pre-existing antibodies and PF4/H complexes.[18]
An important treatment for both HIT and for VITT is IVIgs, a known inhibitor of FcγRIIA.[18] A body of evidence from non-randomised trials and retrospective studies suggests that IVIg may be an effective treatment of VITT, although sometimes not effective as a single agent. Recent articles reported successful treatment of VITT using IVIg, which prevents platelet activation by anti-PF4 antibodies. IVIg treatment parallels emerging experience in the treatment of severe autoimmune HIT in which high-dose IVIg has resulted in rapid increases in platelet count and de-escalation of hypercoagulability.[18] Thus, IVIg is proposed as a therapeutic agent for VITT due to its success in treating autoimmune HIT.
Three cerebral venous sinus thrombosis cases reported by Wolf et al. were managed by heparinisation and endovascular recanalization of the venous sinuses.[18] Under treatment with LMWH, platelet counts normalised within several days, and the patients survived and underwent rehabilitation.[18] In the HEP-COVID randomised clinical trial, therapeutic LMWH dose reduced the composite of thromboembolism and death compared with standard heparin thromboprophylaxis without increasing the frequency of major bleeding among hospitalised patients with COVID-19.[19] The type of anticoagulant may play a role, as a therapeutic dose of LMWH may exert pleiotropic effects such as anti-inflammatory, immunomodulatory, and antiviral effects, in addition to its antithrombotic properties, whereas small-molecule direct oral anticoagulants may lack these properties.[20]
VITT is a novel disease with diverse clinical features, and multiple therapeutic modalities. Non-heparin anticoagulants and immunoglobulins may help treat VITT/TTS.[21] Anticoagulation alone or in combination with eculizumab or IVIg resolved the pathology in three patients.[21] Treatment with fondaparinux, IVIg, and prednisone led to a marked improvement of VITT. Treatments including IVIg, methylprednisolone and direct oral anticoagulant improved VITT gradually.[22] Patients with VITT were treated successfully with IVIg, non-heparin anticoagulants and corticosteroids. Although it is difficult to assess response to treatment in patients with VITT-associated cerebral venous thrombosis in a purely observational study, both non-heparin anticoagulants and IVIg were associated with better outcomes.[23] Both high-dose IVIg (2g/kg body weight over 2 to 5 days) and the potent thrombin inhibitor argatroban are potent agents to block the two fundamental steps triggering TTS: platelet activation (FcγRIIA ligation by PF4-polyanions-IgG complex) and activation of the coagulation cascade (FXII activation by polyphosphates).[23] In severe cases such as severe thrombocytopenia and thrombosis, plasma exchange may be considered along with high-dose IVIg in addition to anticoagulant treatment.
5. Potential alternatives for VITT treatment
Greinacher et al. tested another potential therapeutic, the spleen tyrosine kinase (SYK) inhibitor fostamatinib that is currently used for the treatment of chronic immune thrombocytopenia.[24] The FcγRIIA-dependent signalling mechanism leading to platelet activation in VITT identified by Greinacher et al. provides strong rationale to consider SYK inhibition in the limited therapeutic armamentarium treating VITT and perhaps other forms of autoantibody-mediated thrombosis.[24] Fostamatinib inhibits activation of the Fc receptor by antigen/antibody complexes, and reduced NETosis and platelet activation in ex-vivo COVID-19 studies.[25] Orally administered fostamatinib reduced adverse events and showed a trend toward clinical benefit.[26]
Vayne and collaborators developed a chimeric IgG1 anti-PF4 antibody, 1E12, which strongly mimics “autoimmune” HIT antibodies in terms of specificity and cellular effects, and could be used as a model antibody to study the pathophysiology of VITT.[26] They evaluated the capability of DG-1E12, a deglycosylated form of 1E12 unable to bind Fc receptor, to inhibit cellular activation induced by VITT antibodies. DG-1E12 may allow the development of a new drug neutralising the pathogenic effect of autoimmune anti-PF4 antibodies, such as those associated with VITT.
Afkhami et al. used adenoviral vectors of human and chimpanzee origin, and evaluated Ad-vectored trivalent COVID-19 vaccines expressing spike-1, nucleocapsid, and RNA-dependent RNA polymerase antigens in murine models.[27] Single-dose intranasal immunization, particularly with chimpanzee adenoviral vectored vaccine, is superior to intramuscular immunization in induction of the tripartite protective immunity consisting of local and systemic antibody responses, mucosal tissue-resident memory T cells and mucosal trained innate immunity. They further showed that intranasal immunisation provides protection against the ancestral SARS-CoV-2. Respiratory mucosal delivery of Ad-vectored multivalent vaccine represents an effective next-generation COVID-19 vaccine strategy to induce wide-spread mucosal immunity.[27]
Immunothrombosis is driven by the complement/tissue factor/neutrophil axis, as well as by activated platelets, which can trigger the release of NETs and release further effectors of immunothrombosis, including PF4/CXCL4 and high-mobility box 1 protein (HMGB1).[28] Many of the central effectors of deregulated immunothrombosis, including activated platelets and platelet-derived extracellular vesicles (pEVs) expressing PF4, soluble PF4, HMGB1, histones, as well as histone-decorated NETs, are positively charged and thus bind to heparin. The authors provide evidence that adsorbents functionalised with endpoint-attached heparin efficiently deplete activated platelets, pEVs, PF4, HMGB1 and histones/nucleosomes. They suggested that elimination of central effectors of immunothrombosis, rather than binding directly to pathogens, could be a clinically relevant strategy for mitigating thrombotic complications of sepsis or COVID-19 using heparin-functionalised adsorbents.[28, 29]
6. Conclusions and perspectives
Although scientists around the world have rapidly developed effective vaccines to fight COVID-19, many factors such as the mechanism and risk of VITT remain uncertain. Rare life-threatening thrombotic manifestations appear to occur with all four vaccines, with differences in frequency and mechanism that need further investigation. ChAdOx1 nCoV-19 and in second position, Ad26.COV2-S vaccines showed the most frequent adverse reactions by triggering severe thrombosis. Scientists explained this phenomenon as caused by impurities related to vaccine preparation. VITT is a rare and severe reaction that causes the extreme activation of platelets and coagulation with a high risk of death. Diagnosis should be guided by standardised definitions, and should also take into account early and seemingly atypical presentations. Particularly, identification, quantification and molecular mechanisms of platelets have attracted increasing attention and suggested platelets as new biomarkers for diagnostic and therapeutic strategies in VITT. NETs, cytokines and interleukins are implicated in VITT pathogenesis, indicating intense innate immune activation.
Non-heparin anticoagulants along with IVIg show effectiveness to treat VITT. Important next steps in optimising the management of this novel condition will include defining the optimal duration of anticoagulant treatment and determining the long-term outcomes among affected patients. Other alternatives for vaccination and treatment are always possible. Actually, only vaccines administered intramuscularly and designed to only target the spike protein were experienced. However, there is a pressing need to develop next-generation vaccine strategies for broader and long-lasting protection. Respiratory mucosal delivery of adenoviral vectored multivalent vaccine represents an effective next-generation COVID-19 vaccine strategy to induce all-around mucosal immunity.
Although VITT is successfully diagnosed and several immunologic mechanisms were identified, many questions remain: What is the protein(s) in the vaccine that binds to PF4? What is the precise neoantigen generated when PF4 and vaccine components interact? Do human proteins in the vaccine provoke an immune response? Is the prothrombotic antibody repertoire in VITT limited to PF4, the vaccine and its components, or is there overlap with autoantibodies found in acute COVID-19, autoimmune disease, and other critical illnesses? With ongoing research, other questions are arising, and answering implicates development of vaccines and therapeutics that use adenovirus- and other virus-based vectors.
Thromboembolism remains an extremely rare side effect of COVID-19 vaccination, and the benefits of vaccination against COVID-19 continue to outweigh the risks of side effects. The scientific community should have confidence in the safety of the SARS-CoV-2 vaccines when considering solutions to unwanted side effects. Further analyses based on more detailed reporting of thrombotic adverse events, including patients’ characteristics and comorbidities, may allow for more specific assessment of causality.







