1. Introduction
Currently, single large (over 5 cm) hepatocellular carcinoma (HCC) is classified as early stage according to the criteria of the Barcelona classification of liver cancer (BCLC).[1] However, according to a study prognosis of a single HCC of more than 5 cm was significantly different from that of early stage (BCLC-A) and was similar to that of intermediate stage (BCLC-B).[2, 3] Actually, single large (>5 cm) HCC is beyond the indication liver transplantation (LT) or radiofrequency ablation according to current treatment guideline.[4] In addition, there are not many cases where surgery is practically possible.[5] Therefore, at present, there is no specific treatment method for cases where surgery is difficult.
So, in patients with single large HCC who is not indicated for surgery, transarterial chemoembolization (TACE), or transarterial radioembolization (TARE) may be performed, depending on the extent of vascular invasion, liver function, or various other conditions.[1, 4] TARE using radioactive isotope, with a short half-life and penetration depth, is an intra-arterial brachytherapy characterized by potent anti-cancer effect given by radiation but minimal embolic effect.[6] Previously, there were studies comparing TACE or TARE and surgery in single large HCC in other literature.[5, 7] In addition, according to the existing literature, TARE is not inferior to TACE, and the safety is secured because of the less side effects or shorter hospitalization period.[6, 8-11] But there is lack of study directly comparing the efficacy and safety between TACE and TARE in patients with single large HCC.
Therefore, in this study, we compared the treatment effects, side effects, and safety of TACE and TARE on single large HCC without treatment choice for current treatment methods.
2. Materials and Methods
This study was approved by the Institutional Review Board (IRB) of Severance hospital (approval number: 4-2023-0442). All methods were performed in accordance with the relevant guidelines and regulations of the IRB. All participants provided written informed consent for participation in the study.
From January 2011 to May 2017, patients who received TACE or TARE as a first-line treatment for single large hepatocellular carcinoma (>5 cm) at Severance hospital will be reviewed retrospectively. We excluded subjects who had any of the following: (1) Child-Pugh score over seven; (2) patients with infiltrative tumor; (3) if TACE or TARE was used as an adjunctive therapy other than the primary treatment, or as a follow-up treatment following TACE or TARE during the same hospital stay. Patient groups are classified according to the treatment method for the patients collected within the above-mentioned guided range.
We compare the baseline characteristics of each patient group with the current history, laboratory test results, liver function using Child-Pugh score. Tumor related factor was also compared including size, presence of portal vein invasion or satellite nodule, and tumor markers. Tumor markers included alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II).
As part of the treatment effect analysis in this study, the proportion of patients who underwent resection or LT because curative surgery was possible after the procedure was analyzed for each group.
In addition, we analyzed the treatment response through image work-up 3 months after the procedure to analyze the treatment effect. Treatment response was analyzed using modified Response Evaluation Criteria in Solid Tumors (mRECIST).[12] Complete response (CR), partial response (PR), and stable disease (SD), excluding progressive disease (PD), were defined as disease control and the ratio was calculated separately. If the patient underwent curative resection before image work-up at 3 months or had a follow-up loss, it was excluded from the treatment response analysis.
Survival analysis was performed as the last method to analyze the treatment effect. The survival rate of each patient group is analyzed using Kaplan-Meier curve.[13] Progression free survival and time to secondary therapy will be also calculated by calculating the duration until progression and the second treatment. If the patient has a follow-up loss at Severance, use health insurance data to check for survival.
Nursing records are used to compare the frequency of fever, nausea, vomiting, and abdominal pain during hospitalization to compare adverse effects between treatment groups. Fever was defined as the case where the tympanic membrane temperature was above 37.8 degrees, this is because the nurses notify the doctors from this point on in the institution. Abdominal pain, nausea and vomiting were determined based on patients with Grade 2 or higher of the Common terminology criteria for adverse events (CTCAE) Version 5.0.[14] Not only side effects but also length of hospitalization was calculated and compared for each group.
Additionally, to compare the therapeutic effect as well as the treatment stability, we checked the liver function test day after procedure, three weeks after the procedure and three months after the procedure. At three months, liver function tests as well as Child-Pugh scores were separately calculated. And the difference between the two groups was compared.
All statistical analyses were performed using SPSS version 27 for Windows (SPSS Inc., Chicago, Illinois, USA). Differences in categorical variables between the groups were analyzed using the chi-square test or Fisher’s exact test. Continuous variables were compared using Student’s t-test.[15] Survival statistics were evaluated using Kaplan-Meier method.[16] Statistical significance was set at two-sided p < 0.05.
3. Results
During this period, 386 patients were treated with TARE or TACE as initial treatment and 359 patients were excluded according to the exclusion criteria. After the exclusions were complete, 27 patients were included in the analysis. Of these, 14 patients received TARE and 13 patients received TACE (Fig. 1).
Table 1 presents the baseline characteristics of the subjects in both TARE and TACE groups. There was no difference in subject related-characteristics between the two groups including past history. Pre-procedure laboratory tests have no significant difference except for the platelet count. However, there was no clinical significance because the platelet counts of both groups were within the reference range (219.6 103/μL in TARE group vs. 163.7 103/μL in TACE group, p = 0.021). Tumor size of each group was 7.9 cm in the TARE group and 7.4 cm in the TACE group (p = 0.432), and there was no difference in the mean Child-Pugh score between the two groups (5.1 vs. 5.3, p = 0.557). In pre-procedure imaging, portal vein thrombosis was present in one patient in both groups, and all were segmental branches. There were more satellite nodules in the TARE group (3 patients in the TARE group and 1 patient in the TACE group), but there was no statistical significance (p = 0.315).
Abbreviations: AFP, Alpha-fetoprotein; COPD, Chronic obstructive pulmonary disease; HBV, Hepatitis B virus; HCV, Hepatitis C virus; nBnC, non-HBV and non-HCV; PIVKA-II, Protein induced by vitamin K absence or antagonist-II; TACE, transarterial radioembolization; TARE, transarterial chemoembolization.
The cases which surgical treatment was possible after the procedure were compared between the two groups (Table 2). Resection was performed in 5 patients in both groups (35.7% in TARE vs. 38.5% in TACE, p = 0.883), and LT was performed in 1 patient in both groups (7.1% in TARE vs. 7.7% in TACE, p = 0.957), showing no statistically significant difference.
| Type of surgery | TARE (n=14) | TACE (n=13) | p-value |
|---|---|---|---|
| Resection | 5 (35.7%) | 5 (38.5%) | 0.883 |
| Liver transplantation | 1 (7.1%) | 1 (7.7%) | 0.957 |
The subjects underwent imaging tests at 3 months to evaluate the treatment response, and the results were compared between the two groups (Table 3). In the TACE group, 1 patient underwent curative resection prior to the 3-month response assessment and 1 patient had follow-up loss. These two patients were excluded from the analysis, and only the remaining patients were included in the analysis. CR rate and PR rate were not significantly different between the two groups. However, the SD rate was significantly higher in the TARE group (50.0% in TARE group vs. 9.1% in TACE group, p = 0.038), whereas the PD rate was significantly higher in the TACE group (0.0% in TARE group vs. 45.5% in TACE group, p = 0.009). Therefore, the disease control rate was significantly higher in the TARE group than in the TACE group (100.0% in TARE group versus 54.5% in TACE group, p = 0.009).
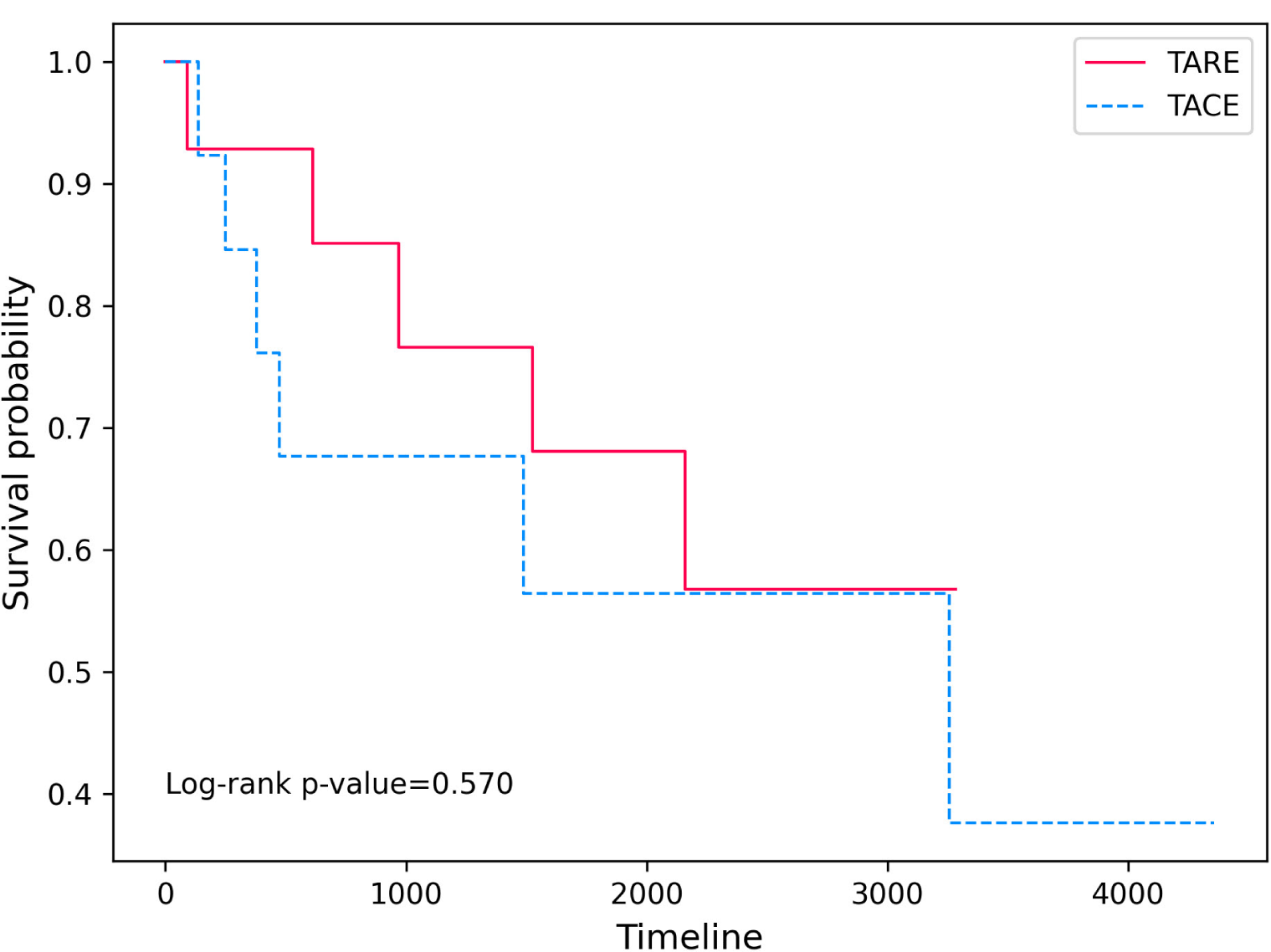
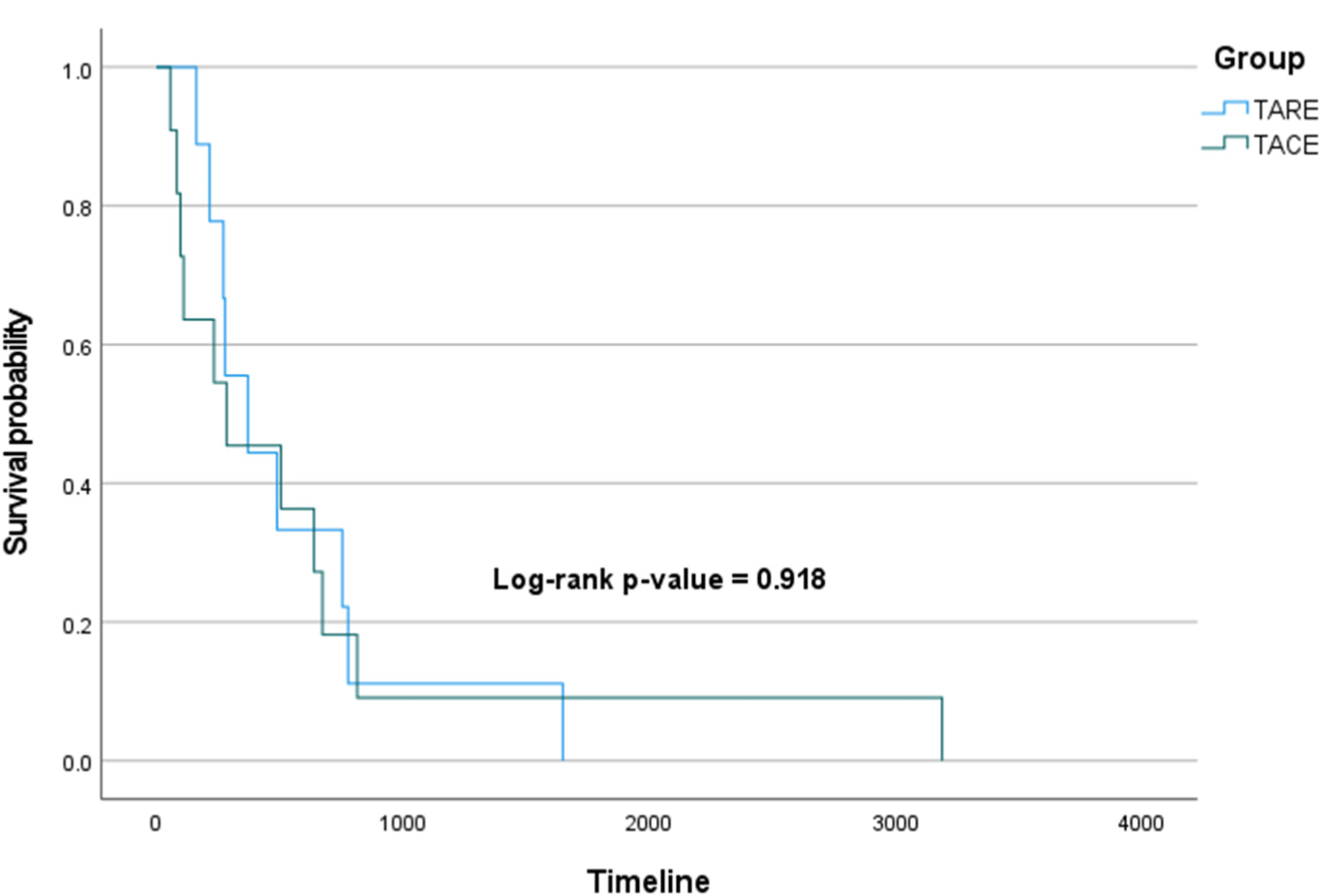
The initial treatment period of the patients included in the analysis was from January 2011 to May 2017, but their follow-up was conducted until February 2023, and OS and PFS were compared between the two groups using survival curves. The Log-rank p-value of OS (Fig. 2) was 0.570, and the Log-rank p-value of PFS (Fig. 3) was 0.918, showing no significant difference between the two groups in both OS and PFS.
To compare safety, side effects and length of hospital stay were compared between the two groups (Table 4). In addition, several laboratory tests related to the liver were compared the day after the procedure, 3 weeks after the procedure, and 3 months after the procedure (Table 5). There was no significant difference between the two groups in CTCAE grade 2 or higher abdominal pain and nausea. However, fever did not occur in the TARE group, but occurred in 10 patients (76.9%) in the TACE group, showing a statistically significant difference (p < 0.001). The length of hospital stay was 3.79 days on average in the TARE group, but 5.92 days on average in the TACE group, which was also statistically significant (p = 0.003). As post-procedure laboratory tests, AST, ALT, total bilirubin, and serum albumin levels were checked. On the first day after the procedure, there was no significant difference in the AST level among liver enzymes (p = 0.052). But the average ALT level in the TARE group was 54.6 IU/L, whereas in the TACE group it was 221.0 IU/L, which was higher in the TACE group and statistically significant (p = 0.045). On the same day, the total bilirubin level was statistically significantly lower in the TARE group (0.76 mg/dL in TARE group vs. 1.12 mg/dL in TACE group, p = 0.028), and the serum albumin level was significantly lower in the TACE group(3.77 mg/dL in TARE group vs. 3.47 mg/dL in TACE group, p = 0.023), but it was within the normal range and had no clinical significance. At 3 weeks after the procedure, there was no significant difference in other laboratory tests, but the serum albumin level was found to be significantly lower in the TACE group as on the 1st day (4.05 mg/dL in TARE group vs. 3.53 mg/dL in TACE group, p = 0.005). However, it was also within the normal range and had no clinical significance. Likewise, at 3 months after the procedure, only the serum albumin level was different statistically but not clinically significant (4.19 mg/dL in TARE group vs. 3.80 mg/dL in TACE group, p = 0.035). In terms of Child-Pugh score, the TARE group averaged 5 points and the TACE group averaged 5.92 points, showing a higher tendency for the TACE group, but there was no statistical significance (p = 0.064).
4. Discussion
Although there have been studies comparing TARE with other treatment modalities or comparing TARE with TACE in various type of HCC, this study is the first to compare TACE and head-to-head in single large HCC. We could not demonstrate significant difference in overall survival or progression free survival between treatment modalities. However, although there are limitations due to the small number of subjects, we confirmed that the TARE group showed a superior disease control rate compared to TACE at three months after treatment. And we also proved that TARE was superior in terms of side effects and length of hospitalization despite having the same baseline characteristics.
We hypothesized that TARE would be superior to TACE in terms of efficacy and safety in single large HCC. This is because in previous studies, TARE showed comparable outcomes to resection for single large HCC, and TARE is known to be more effective and safer than TACE in several types of HCC.[5, 9, 17-19] In our results, TARE showed a better treatment response at three months and was also superior in terms of side effects. However, this has not been demonstrated in OS and PFS, which can be considered for the following reasons. As will be mentioned later, it is possible that this is due to the small number of subjects, which is the biggest drawback of this study.[20] However, it is unreasonable to simply apply that reason because there were significant results in the treatment response and side effects at 3 months. One possibility is that there was no benefit to OS as a result of TARE-induced long-term toxicity and complications.[21, 22] And since the response by mRECIST is based on the radiologic image, it is only an indirect reflection of the tumor response, and therefore the behavior of the actual tumor may be different.[12, 23] However, these are only hypotheses and we are not sure as there is no head-to-head comparison with TACE.
Although excellence in survival was not demonstrated, our study is meaningful in the following aspects. A high disease control rate over a specific period of time reduces the need for other treatments. In addition, there are few side effects after the procedure, and the average hospitalization length is shortened by about two days. Although medical costs vary from country to country, these results suggest that TARE has an advantage in terms of medical costs.[24, 25]
Although there are positive results, this study has many limitations. First of all, since this study was conducted at one institution, it is highly likely that there would be various biases such as the type of patient group or the level of procedure.[26] Second, the number of subjects was very small, so some results were different between the two groups, but statistical significance was not obtained with a slight difference.[20] And for some results, the possibility that the p-value was exaggerated because the result of one group was measured as 0 cannot be excluded.[20, 26] Third, this study was a retrospective design, and although there was little difference in baseline characteristics between the two groups, the level of evidence cannot be considered high.[27] Lastly, the subjects of this study were patients from about 12 years ago at the longest and about six years ago at the shortest time, and patients whose treatment time has elapsed considerably, and may show different treatment tendencies from the present.
Therefore, because of these limitations, it is thought that the following additional efforts are needed to reconsider the reliability of this study. A large number of subjects must be collected to increase the reliability of research results and to obtain more results[20]. And Multicenter studies are needed to reduce bias. In addition, a randomized controlled study is necessary to increase the level of evidence[27], but it is difficult in reality. This is because there are studies that, although not limited to single large HCC, suggest that TARE is superior to TACE, and the BCLC guideline revised in 2022 recommends TARE treatment for single large HCC less than 8 cm.[1] Therefore, if various results are collected and meta-analysis is performed, the level of evidence can be increased.[27]
5. Conclusion
This single center retrospective study has several limitations including that the number of subjects was small. Nevertheless, we demonstrated that TARE can be more advantageous than TACE in terms of disease control rate, hospital stay, and side effects in single large HCC. However, more follow-up studies are needed to increase reliability.







