1. Introduction
Pharmacovigilance and medical product safety issues are important for all global citizens. Previous studies have analyzed the disaster caused by thalidomide in 1961 which exposed pregnancy mothers to thalidomide and leaded many thousands of infants with congenitally malformation.[1] In 1963, the 16th World Health Assembly was determined to create the World Health Organization (WHO) Pilot Research Project for International Drug Monitoring to develop and understand adverse effects of medical products.[2] In 1968, this pilot research project developed into the WHO Programme for International drug Monitoring (VigiBase) coordinated by the Uppsala Monitoring Center (Uppsala, Sweden) and expanded into an organization with 153 member countries, 22 associates, and many regional reporting centers, as of March 2022.[3]
The main goal of VigiBase is detection, understanding, assessment, and prevention of adverse effects of medical products (herbals, complementary medicines, biologic products, blood products, medical devices, and vaccines). Adverse drug reactions are calculated and examined the individual case safety reports (ICSRs) in the WHO international pharmacovigilance database (Fig. 1).[4]
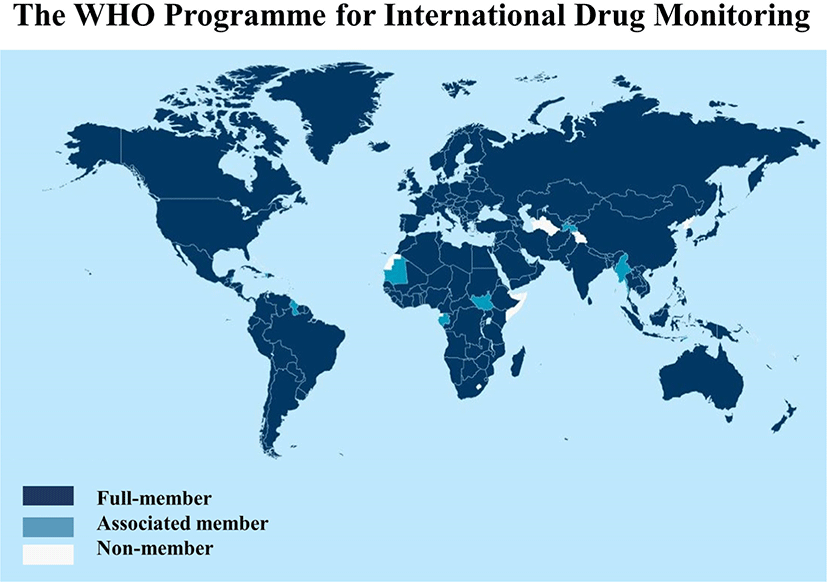
2. Full members of the WHO programme for international drug monitoring (year they joined)
Afghanistan (2016), Albania (2020), Algeria (2021), Andorra (2008), Angola (2013), Argentina (1994), Armenia (2001), Australia (1968), Austria (1991), Azerbaijan (2018), Bangladesh (2014), Barbados (2008), Belarus (2006), Belgium (1977), Benin (2011), Bhutan (2014), Bolivia (Plurinational State of) (2013), Bosnia and Herzegovina (2019), Botswana (2009), Brazil (2001), Brunei Darussalam (2005), Bulgaria (1975), Burkina Faso (2010), Burundi (2022), Cabo Verde (2012), Cambodia (2012), Cameroon (2010), Canada (1968), Central African Republic (2022), Chad (2018), Chile (1996), China (1998), Colombia (2004), Congo (2021), Costa Rica (1991), Côte d’Ivoire (2010), Croatia (1992), Cuba (1994), Cyprus (2000), Czechia (1992), Democratic Republic of the Congo (2010), Denmark (1971), Dominican Republic (2020), Ecuador (2017), Egypt (2001), El Salvador (2017), Eritrea (2012), Estonia (1998), Eswatini (2015), Ethiopia (2008), Fiji (1999), Finland (1974), France (1986), Gambia (2021), Georgia (2018), Germany (1968), Ghana (2001), Greece (1990), Guatemala (2002), Guinea (2013), Guinea-Bissau (2022), Honduras (2020), Hungary (1990), Iceland (1990), India (1998), Indonesia (1990), Iran (Islamic Republic of) (1998), Iraq (2010), Ireland (1968), Israel (1973), Italy (1975), Jamaica (2012), Japan (1972), Jordan (2002), Kazakhstan (2008), Kenya (2010), Kuwait (2021), Kyrgyzstan (2003), Lao People’s Democratic Republic (2015), Latvia (2002), Lebanon (2021), Liberia (2013), Libya (2021), Lithuania (2005), Luxembourg (2020), Madagascar (2009), Malawi (2019), Malaysia (1990), Maldives (2016), Mali (2011), Malta (2004), Mauritius (2014), Mexico (1999), Mongolia (2021), Montenegro (2009), Morocco (1992), Mozambique (2005), Namibia (2008), Nepal (2006), Netherlands (1968), New Zealand (1968), Nicaragua (2020), Niger (2012), Nigeria (2004), North Macedonia (2000), Norway (1971), Oman (1995), Pakistan (2018), Panama (2016), Papua New Guinea (2018), Paraguay (2018), Peru (2002), Philippines (1995), Poland (1972), Portugal (1993), Republic of Korea (1992), Republic of Moldova (2003), Romania (1976), Russian Federation (1998), Rwanda (2013), Saint Vincent and the Grenadines (2020), Saudi Arabia (2009), Senegal (2009), Serbia (2000), Sierra Leone (2008), Singapore (1993), Slovakia (1993), Slovenia (2010), South Africa (1992), Spain (1984), Sri Lanka (2000), Sudan (2008), Suriname (2007), Sweden (1968), Switzerland (1991), Syrian Arab Republic (2018), Thailand (1984), Togo (2007), Tunisia (1993), Türkiye (1987), Uganda (2007), Ukraine (2002), United Arab Emirates (2013), United Kingdom of Great Britain and Northern Ireland (1968), United Republic of Tanzania (1993), United States of America (1968), Uruguay (2001), Uzbekistan (2006), Venezuela (Bolivarian Republic of) (1995), Viet Nam (1999), Yemen (2022), Zambia (2010), and Zimbabwe (1998)
3. Associate members of the WHO programme for international drug monitoring
Antigua and Barbuda, Bahamas, Bahrain, Belize, Dominica, Gabon, Grenada, Guyana, Haiti, Lesotho, Mauritania, Myanmar, Qatar, Saint Kitts and Nevis, Saint Lucia, South Sudan, Tajikistan, Timor-Leste, Anguilla, British Virgin Islands, Montserrat, and Zanzibar.
4. Data source
VigiBase contains 24 million deduplicated ICSRs, some which initiate from November 14, 1967. Each ICSR consists of data reports, country of origin (Americas, Europe, Australia, Asia, and Africa), qualification of reporters (health care professional and non-health care professional), patient data (age and sex), data of drugs (indication for the drug, dosage, route of administration, and start and end dates), adverse drug reaction data reported by Medical Dictionary for Regulatory Activities (MedDRA) classification terms (onset data, end data, seriousness, and final outcome), and additional information (concurrent use of other medication).[2, 5] The MedDRA is a hierarchical terminology classified into five levels (Lowest Level Terms, Preferred Terms, High Level Terms, High Level Group Terms, and System Organ Classes).[6] According to the WHO and Uppsala Monitoring Center policy, all patient data are anonymized and publication of data on individual patients is not permitted.[2, 3, 7] Serious adverse drug reactions are defined as death, life-threatening events, hospitalization (initial or prolonged), disability (leading to persistent or clinically), and clinically serious situations judged by physician reporting the case.
5. Data limitations
VigiaBase has some limitations. First, as the data is collected from 153 member countries, 22 associates, and many regional reporting centers, the quality and diversity of the data varies depending on the reporting method of each country.[8] Certain countries report only suspected adverse drug reaction with potential causal association between drug and ICSR, while the United States reports all ADRs, even if they are not linked to drug use.[5] Secondly, while a small proportion of reports have been generated only from clinical trials, most reports are based on multiple sources (physicians, pharmacists, health care professionals, and patients), which results high heterogeneity of the dataset.[9]
6. Signal detection
VigiBase is based on a case-non-case analysis (disproportionality analysis) in order to generate and report suspected adverse reaction signals in the entire database (Fig. 2). These analyses, with Information component (IC) value used the Bayesian neural network method developed and validated by Uppsala Monitoring Centre[1, 10], can be used to compare proportion of specific adverse reaction for selected drug with the proportion of the same adverse drug reaction for control drugs (entire database). The statistical formula is log2 ([Nobserved+0.5]/[Nexpected+0.5], where Nobserved is the actual number of ICSRs and Nexpected is the expected number of ICSRs. Nexpected is calculated by (Ndrug * Neffect)/Ntotal, where Ndrug is the number of ICSRs for the drug, regardless of adverse drug reaction, Neffect is the number of ICSRs for the adverse drug reaction, regardless of the drug, and Ntotal is the total number of ICSRs in entire database. IC0.25 is the lower end of a 95% credibility interval and the positive value (IC0.25 >0) is considered to be the statistically significant signal detection validated by Uppsala Monitoring Center.[1] This method of using probabilistic logic in information theory was used to identify and find adverse drug reaction signals by a regulatory agency and to avoid false positive signals between a selected drug and a common signal from the entire database.
7. Conclusions
The WHO Global ICSR Database System (VigiBase) provides an appropriate and strong reference of adverse reactions in specific drugs and a novel method to identify specific features of adverse drug reactions. I believe that future findings through VigiBase may contribute to a more comprehensive understanding of adverse reactions in specific drugs, improvement of public and global health, and prevention of global drug disaster such as thalidomide.







