State-of-the-Art Review
Extrapulmonary clinical manifestations of COVID-19: an umbrella review of meta-analysis
Young Joo Han
1,#, Keum Hwa Lee
2,#, Jae-Young Lee
3, Oh Youn Kim
3, Seungeon Moon
3, Sunghyuk Kim
3, Seokhyeon Ryu
3, Dongsu Lee
3, Jae yun Kim
3, Taeyeon Kim
3, Song Lee
3, Seok-Joo Bae
3, Minho Lee
3, Jaewon So
3, Jae Il Shin
2,*
1Department of Pediatrics, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea
2Department of Pediatrics, Yonsei University College of Medicine, Seoul, Republic of Korea
3Yonsei University College of Medicine, Seoul, Republic of Korea
*Correspondence: Jae Il Shin, Tel: +82-2-2228-2050, E-mail:
shinji@yuhs.ac
# These authors contributed equally to this work
© Copyright 2022 Life Cycle. This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: Jan 04, 2022; Revised: Feb 20, 2022; Accepted: Mar 09, 2022
Published Online: Mar 23, 2022
Abstract
Since its emergence, the coronavirus disease 2019 (COVID-19), compared to other coronavirus diseases throughout history, has generated large waves of infection with symptoms such as fever, chills, cough, fatigue, headache, and sore throat appeared. Despite many efforts and gains through meta-analyses of the symptoms of COVID-19 thus far, there has been a need of summarized concise information for clinicians. This review presents a summary of recent meta-analyses by categorizing the various extra-pulmonary clinical symptoms of COVID-19 according to organ systems. The article focuses on six groups: generalized symptoms such as fever (prevalence rate, 71% to 91%), fatigue (prevalence rate, 32% to 58%), and myalgia (20% to 33%), neurologic symptoms (headache [8% to 44%], dizziness [7% to 10%], confusion [5% to 11%], and impaired consciousness [2% to 7%]), psychiatric symptoms (anxiety or agitation [15% to 45%], post-traumatic stress disorder [20% to 41%], sleep disorder [35% to 82%], and depression [21% to 42%]), cardiovascular symptoms (chest pain [8% to 13%], palpitation [9.3%], arrhythmia [1.9%], arrhythmias [18.4%], angina [10.2%], myocardial injury [10.3%], myocardial infarction [3.5%], and acute heart failure [2.0%]), gastrointestinal symptoms (anorexia [20.0% to 27%], nausea or vomiting [5% to 10%], diarrhea [7% to 17%], and abdominal pain [4% to 9%]), and olfactory, gustatory/oral, and ocular symptoms (ocular symptoms [11.2%], olfactory dysfunction [38% to 53%], and gustatory dysfunction [7% to 37%]). The aim of this study is to provide clinicians concise information of the various extra-pulmonary clinical symptoms of COVID-19 and promote the understanding of administrators who set appropriate quarantine policies.
Keywords: COVID-19; Extrapulmonary symptoms; Olfactory dysfunction; Gustatory dysfunction; Meta-analysis
1. Introduction
In December 2019, the first few cases of pneumonia with an unidentified origin were reported in Wuhan, Hubei Province, China.[1] The World Health Organization declared a public health emergency caused by this disease called coronavirus disease 2019 (COVID-19) in March 2020.[2, 3] As of March 15, 2022, 455,565,230 cases and 6,039,440 deaths have been reported globally.[2, 3]
There is a wide understanding that COVID-19 is less pathogenic but highly contagious compared to other human coronavirus diseases throughout human history.[4] In addition, the transmission rate was highest before or immediately after symptoms such as fever, chills, cough, fatigue, headache, and sore throat appeared in patients.[2, 3] Therefore, rapid diagnostic evaluation based on symptoms is crucial in order to minimize the risk of transmission of COVID-19.[4] However, numerous meta-analyses to date have shown that the symptoms of COVID-19 are highly variable across each organ system.
This review article summarized meta-analyses of the various extrapulmonary clinical symptoms of COVID-19, aiming to provide concise information for clinicians to accurately determine infection and transmission of COVID-19 and for administrators to set appropriate quarantine policies.
2. Symptoms of COVID-19
2.1. Generalized symptoms
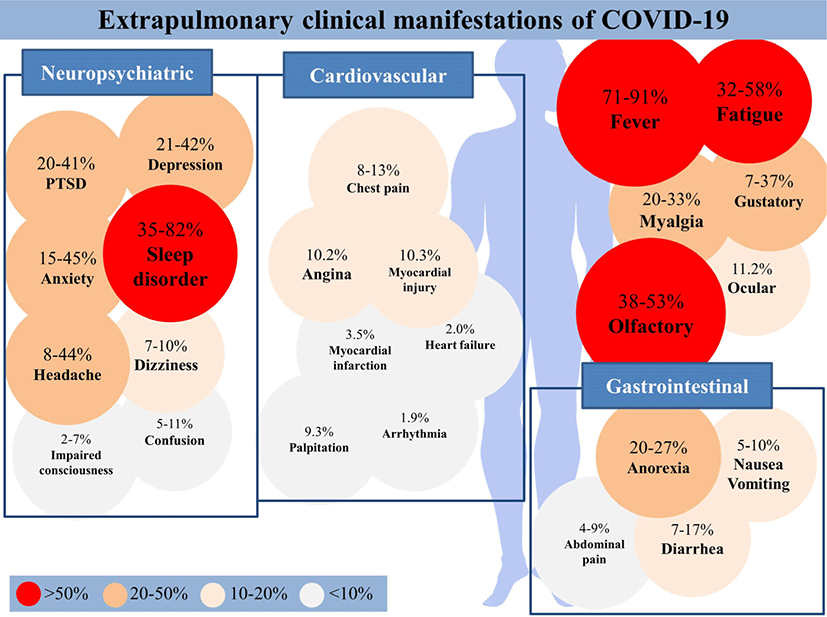
Fever, fatigue, and myalgia were the most commonly reported systemic symptoms of COVID-19 (Fig. 1).[5-14] Among them, fever was the most prevalent symptom, showing prevalence rates from 71% to 91%, and was observed in relevant studies.[5-8, 15] Fatigue and myalgia were also frequently reported in most studies, each showing prevalence rates from 32% to 58% and 20% to 33%, respectively. [7-17]. Studies reporting generalized symptoms are summarized in Table 1.
Table 1.
Meta-analyses reporting generalized symptoms in patients with COVID-19
|
First author (date of publication) |
Study period |
Number of studies/participants |
Prevalence of symptoms (95% CI) |
|
Groff (Oct 13, 2021) |
Dec 2019 to Mar 2021 |
57/250,351 |
Fatigue or weakness 37.5% (IQR, 25.4% to 54.5%) |
|
Lopez-Leon (Aug 09, 2021) |
Dec 2019 to Jan 2021 |
15/47,910 |
Fatigue 58% (42% to 73%) |
|
Misra (Dec 07, 2021) |
Dec 2019 to Dec 2020 |
350/145,721 |
Fatigue 32%
Myalgia 20%
Myalgia or fatigue 25 to 37% |
|
Rogers (Jun 3, 2021) |
Dec 2019 to Jul 2020 |
147/99,905 |
Fatigue 37.8% (31.6% to 44.4%)
Weakness 40.0% (27.9% to 53.5%)
Myalgia 25.1% (19.8% to 31.3%) |
|
Mirmoeeni (Apr 24, 2021) |
Dec 2019 to Apr 2020 |
16/4,754 |
Fatigue 39.27% (30.92% to 47.61%) |
|
Li (Aug 13, 2020) |
Ja 2020 to Apr 2020 |
212/281,461 |
Fever 78.8% (76.2% to 81.3%)
Malaise 37.9% (29.5% to 47.1%) |
|
Ahmed (May 08, 2020) |
Dec 2019 to Mar 2020 |
13/24,677 |
Fever 71% (China vs. others 85% vs 53%, p < 0.001) |
|
Kumar (Apr 21, 2020) |
Jan 2020 to Mar 2020 |
58/6,892 |
Fever 83.4% Fatigue 33.8%
Association of high fever (>39 °C) and severity: OR 1.59 (1.10 to 2.30) |
|
Manabe (Jun 28, 2020) |
Dec 2019 to Feb 2020 |
13/2,397 |
Fever 82.46% (69.62% to 95.30%)
Fatigue 41.11% (25.08% to 57.13%)
Myalgia 20.10% (12.08% to 28.11%) |
|
Zhu (Apr 13, 2020) |
Jan 2020 to Feb 2020 |
38/3,062 |
Fever 80.4% (73.0% to 86.9%)
Fatigue 46.0% (38.2% to 54.0%)
Myalgia 33% (26.0% to 40.5%) |
|
Yang (Mar 12, 2020) |
Dec 2019 to Feb 2020 |
7/1,576 |
Fever 91.3% (86% to 97%)
Fatigue 51.0% (34% to 68%) |
Download Excel Table
Ahmed et al. found that patients in China had a higher rate of fever compared to patients in other countries [6]. This is consistent with the findings of Manabe et al., which showed that the expected frequency of fever decreased as COVID-19 spread in China [5].
In this meta-analysis, the estimated frequency of fever was highest in the study group where most patients lived in Wuhan, Hubei Province, and were directly exposed to the Hunan seafood wholesale market, compared to the study group in which patients lived in Hubei Province but were not directly exposed to the market or lived outside Hubei Province.[5-13] Similarly, in a study performed by Zhu et al., a higher frequency of fever was found in patients in Hubei province (87%) than in patients living outside Hubei province (76%).[8] These results suggest that the frequency of fever decreased as COVID-19 spread sequentially from Wuhan to Hubei Province, other parts of China, and worldwide.
Fatigue also showed a similar tendency. In a previous study, the prevalence of fatigue was highest in patients directly exposed to the Hunan seafood wholesale market (70%), followed by patients living in Hubei Province (56%) and patients living outside Hubei Province (22%).[5] In another previous study, patients from Hubei province (62%) showed more symptoms of fatigue than patients outside Hubei province (36%).[8]
2.2. Neurologic symptoms
Headache was the most common neurologic symptom, with a prevalence rate of 8% to 44%. Neurological symptoms such as dizziness (prevalence rate, 7% to 10%), confusion (5% to 11%), and impaired consciousness (2% to 7%) were also reported with considerable prevalence (Table 2).[9-12, 16, 17]
Table 2.
Meta-analyses reporting neurologic symptoms in patients with COVID-19
|
First author (date of publication) |
Study period |
Number of studies/participants |
Prevalence of symptoms (95% CI) |
|
Groff (Oct 13, 2021) |
Dec 2019 to Mar 2021 |
57/250,351 |
Memory deficits 18.6% (IQR, 17.3% to 22.9%
Cognitive impairment 17.1% (IQR, 14.1% to 30.5%)
Headache 8% (IQR, 1.9% to 13.9%) |
|
Lopez-Leon (Aug 09, 2021) |
Dec 2019 to Jan 2021 |
15/47,910 |
Headache 44% (13% to 78%) |
|
Misra (Dec 07, 2021) |
Dec 2019 to Dec 2020 |
350/145,721 |
Headache 13% (12% to 15%)
Dizziness 7% (5% to 8%)
Confusion 11% (7% to 16%)
Impaired consciousness 7% (5% to 10%) |
|
Rogers (Jun 3, 2021) |
Dec 2019 to Jul 2020 |
147/ 99,905 |
Headache 20.7% (16.1% to 26.1%)
Altered mental status 8.2% (4.4% to 14.8%) |
|
Wang (Jun 11,2020) |
Dec 2019 to May 2020 |
41/3,837 |
Headache 9.2% (7.2 to 11.2)
Dizziness 10.0% (5.9% to 14.2%)
Confusion 5.2% (1.7% to 12.2%) |
|
Collantes (Jul 15,2020) |
Jan 2020 to Apr 2020 |
35/6,335 |
Headache 12% (10% to 14%)
Dizziness 8% (5% to 12%)
Confusion 5% (2% to 14%) |
|
Abdullahi (Jun 26, 2020) |
Dec 2019 to Apr 2020 |
51/11,069 |
Headache 12% (9% to 15%)
Dizziness 10% (3% to 19%)
Impaired consciousness 2% (1% to 2%) |
|
Pinzon (May 29, 2020) [23] |
Jan 2020 to Apr 2020 |
33/7,559 |
Headache 10.9% (8.62% to 13.51%)
Dizziness 8.7% (5.02% to 13.43%)
Impaired consciousness 3.8% (0.16% to 12.04%) |
|
Rogers (May 18, 2020) |
Jan 2020 to Apr 2020 |
7/3,559 |
Altered consciousness: 21% of patients who subsequently died |
Download Excel Table
2.3. Psychiatric symptoms
The prevalence of anxiety or agitation ranged from 15% to 45%. [11, 12, 18-21] Furthermore, the prevalence of post-traumatic stress disorder, sleep disorder, and depression were 20% to 41%[19, 20], 35% to 82%[19-21], and 21% to 42%[19-21], respectively (Table 3).
Table 3.
Meta-analyses reporting psychiatric symptoms in patients with COVID-19
|
First author (date of publication) |
Study period |
Number of studies/participants |
Prevalence of symptoms (95% CI) |
|
Khraisat (Oct 28, 2021) |
Dec 2019 to Aug 2021 |
27/9,605 |
PTSD 20% (16% to 24%)
Anxiety 22% (18% to 27%)
Depression 21% (16% to 28%)
Sleeping disorder 35% (29% to 41%) |
|
Groff (Oct 13, 2021) |
Dec 2019 to Mar 2021 |
57/250,351 |
Difficulty in concentrating 23.8% (IQR, 20.4% to 25.9%) |
|
Lopez-Leon (Aug 09, 2021) |
Dec 2019 to Jan 2021 |
15/47,910 |
Attention disorder 27% (19% to 36%) |
|
Misra (Dec 07, 2021) |
Dec 2019 to Dec 2020 |
350/145,721 |
Agitation 45% (3% to 93%) |
|
Dong (Jun 2, 2021) |
Jan 2020 to Oct 2020 |
44/8,587 |
Anxiety 16.6% (10.1% to 23.1%)
Depression 37.7% (29.3% to 46.2%)
Post-traumatic stress disorder 41.5% (9.3% to 73.7%)
Insomnia 68.3% (48.6% to 88.0%)
Somatization 36.5% (20.2% to 52.8%)
Fear 47.6% (9.4% to 85.7%) |
|
Rogers (Jun 3, 2021) |
Dec 2019 to Jul 2020 |
147/99,905 |
Anxiety 15.9% (5.6% to 37.7%) |
|
Krishnamoorthy (Aug 13, 2020) |
Jan 2020 to Apr 2020 |
50/171,571 |
Depression in COVID-19 patients: 42%
Depression in general population: 24%
Anxiety: 37%/26%
Poor sleep quality: 82%/34% |
|
Rogers (May 18, 2020) |
Jan 2020 to Apr 2020 |
7/3,559 |
Delirium: 65% in intensive care unit patients
Agitation: 69% in intensive care unit patients |
Download Excel Table
2.4. Cardiovascular symptoms
The prevalence of chest pain ranged from 8% to 13%.[9] Other presented cardiovascular symptoms were palpitation (9.3%), arrhythmia (1.9%), arrhythmias (18.4%), angina (10.2%), myocardial injury (10.3%), myocardial infarction (3.5%), and acute heart failure (2.0%) (Table 4). [9, 13, 22, 23]
Table 4.
Meta-analyses reporting cardiovascular symptoms in patients with COVID-19
|
First author (date of publication) |
Study period |
Number of studies/participants |
Prevalence of symptoms (95% CI) |
|
Groff (Oct 13, 2021) |
Dec 2019 to Mar 2021 |
57/250,351 |
Chest pain 13.3% (IQR, 8.8% to 17.8%)
Palpitation 9.3% (IQR, 6.0% to 10.8%) |
|
Mirmoeeni (Apr 24, 2021) |
Dec 2019 to Apr 2020 |
16/4,754 |
Chest pain 7.80% (2.74% to 12.86%)
Arrhythmias 1.92% (0.35% to 3.50%) |
Download Excel Table
2.5. Gastrointestinal symptoms
The prevalence of anorexia ranged from 20% to 27%.[24-28] In addition, the prevalence of nausea or vomiting, diarrhea, and abdominal pain were 5% to 10%, 7% to 17%, and 4% to 9%, respectively (Table 5).[15, 24-33]
Table 5.
Meta-analyses reporting generalized symptoms in patients with COVID-19
|
First author (date of publication) |
Study period |
Number of studies/participants |
Prevalence of symptoms (95% CI) |
|
Yusuf (Apr 19, 2021) |
Dec 2019 to Jan 2021 |
22 studies |
Prolonged nausea 3.23% (0.54% to 16.53%)
Persistent vomiting 3.19% (1.62% to 6.17%)
Prolonged diarrhea 4.12% (1.07% to 14.64%)
Prolonged abdominal pain 1.68% (0.84% to 3.32%)
Persistent loss of appetite 4.41% (1.91% to 9.94%) |
|
Shehab (Mar 4, 2021) |
Dec 2019 to Dec 2020 |
158/78,798 |
Diarrhea 16.5% (14.2% to 18.4%)
Nausea 9.7% (9.0% to 13.2%) |
|
Elshazli (Feb 23, 2021) |
Dec 2019 to Jul 2020 |
125/25,252 |
Anorexia 19.9% (15.85% to 23.95%)
Diarrhea 13.2% (11.50% to 14.90%)
Nausea 10.3% (8.00% to 12.60%)
Hematemesis 9.1% (24.90% to 43.10%)
Vomiting 6.30% (5.05% to 7.55%)
Abdominal pain 5.60% (3.95% to 7.25%) |
|
Dorrell (Nov 21, 2020) |
Dec 2019 to May 2020 |
108/17,776 |
Anorexia 21% (15% to 27%)
Diarrhea 13% (11% to 16%)
Nausea or vomiting 8% (6% to 11%)
Abdominal pain 4% (2% to 6%) |
|
Tariq (Jun 10, 2020) |
Dec 2019 to May 2020 |
78/12,797 |
Diarrhea 12.4% (8.2% to 17.1%)
Nausea/vomiting 9.0% (5.5% to 12.9%)
Loss of appetite 22.3% (11.2% to 34.6%)
Abdominal pain 6.2% (2.6% to 10.3%) |
|
Li (Aug 13, 2020) |
Jan 2020 to Apr 2020 |
212/281,461 |
Diarrhea 9.5% (7.8% to 11.5%)
Abdominal pain 4.5% (3.3% to 6.2%)
Vomiting 4.7% (3.8% to 5.8%) |
|
Rokkas (Jun 6, 2020) |
Dec 2019 to Apr 2020 |
37/5,601 |
Diarrhea 9.8% (6.4% to 14.7%)
Nausea/vomiting 10.4% (4.8% to 12.1%)
Abdominal discomfort/pain 6.9% (4.8% to 12.1%) |
|
Mao (May 12, 2020) |
Jan 2020 to Apr 2020 |
35/6,686 |
Diarrhea 9% (6% to 12%)
Nausea/vomiting 6% (5% to 9%)
Loss of appetite 21% (9% to 44%)
Abdominal pain 3% (2% to 5%) |
|
Parasa (Jun 11, 2020) |
Dec 2019 to Mar 2020 |
29/4,805 |
Diarrhea 7.4% (4.3% to 12.2%)
Nausea/vomiting 4.6% (15.3% to 25.6%) |
|
Suresh Kumar (May 25, 2020) |
Dec 2019 to Mar 2020 |
17/2,477 |
Abdominal pain 2.7%
Diarrhea 7.8%
Nausea/vomiting 5.6% |
|
Wang (May 12, 2020) |
Dec 2019 to Mar 2020 |
21/3,024 |
Diarrhea 9.1% (6.3% to 11.9%)
Nausea/vomiting 5.2% (3.5% to 7.0%)
Abdominal pain 3.5% (1.7% to 5.4%) |
|
Cheung (Apr 03, 2020) |
Dec 2019 to Mar 2020 |
60/4,243 |
Loss of appetite 26.8% (16.2% to 40.8%)
Nausea/vomiting 10.2% (6.6% to 15.3%)
Diarrhea 12.5% (9.6% to 16.0%)
Abdominal pain/discomfort 9.2% (5.7% to 14.5%) |
Download Excel Table
2.6. Olfactory, Gustatory/Oral, and Ocular symptoms
Studies reporting olfactory, gustatory, and ocular symptoms are summarized in Table 6. Olfactory (38% to 53%) and gustatory dysfunction (7% to 37%) were common symptoms reported in many COVID-19 patients.[12, 24, 34-41] The prevalence of ocular symptoms was 11.2%.[41] The prevalence of olfactory/gustatory dysfunction was higher in the cases where objective methods were used for the evaluation of olfactory/gustatory function compared to cases where subjective methods were used (77% vs. 45%).[40] In a study comparing patients with severe COVID-19 symptoms with those with mild or moderate COVID-19 symptoms, 31% of patients with severe COVID-19 and 67% of patients with mild or moderate COVID-19 developed olfactory/gustatory symptoms.[39] Two possible causes can be suggested: olfactory/gustatory symptoms were not documented as considered insignificant in patients with severe symptoms, or in mild-to-moderate COVID-19, nasal-centric viral spread, causing olfactory/gustatory symptoms, may predominate, whereas in severe COVID-19, pulmonary-centric viral transmission may predominate.[39]
Table 6.
Meta-analyses reporting olfactory, gustatory/oral, and ocular symptoms in patients with COVID-19
|
First author (date of publication) |
Study period |
Number of studies/participants |
Prevalence of symptoms (95% CI) |
|
Yusuf (Apr 19, 2021) |
Dec 2019 to Jan 2021 |
22 studies |
Dysgeusia 7.04% (5.96% to 8.30%) |
|
Mutiawati (Jan 21, 2021) |
Dec 2019 to Nov 2020 |
107/32,142 |
Anosmia 38.2% (36.5% to 47.2%)
Dysgeusia 36.6% (35.2% to 45.2%) |
|
Hoang (Sep ,2020) |
Dec 2019 to Sep 2020 |
14/8,871 |
Gustatory dysfunction 47.0% (17.3% to 76.8%)
Olfactory dysfunction 45.7% (22% to 69.3%) |
|
Rogers (Jun 3, 2021) |
Dec 2019 to Jul 2020 |
147/99,905 |
Anosmia 43.1% (35.2% to 51.3%)
Dysgeusia 37.2% (29.8% to 45.3%) |
|
Elshazli (Feb 23, 2021) |
Dec 2019 to Jul 2020 |
125/25,252 |
Dysgeusia/ageusia 15.40% (6.20% to 24.60%) |
|
Amorim Dos Santos (Sep 11, 2020) |
Dec 2019 to JUNE 2020 |
40/10,228 |
Gustatory impairment 45% (34% to 55%)
Dysgeusia 38%
Hypogeusia 35%
Ageusia 24% |
|
Ibekwe (Sep 11, 2020) |
Dec 2019 to May 2020 |
27/19,424 |
Loss of smell 48.47% (33.78% to 63.29%)
Loss taste 41.47% (3.13% to 31.03%) |
|
Chi (Aug 22, 2020) |
Dec 2019 to May 2020 |
12/1,739 |
Olfactory and gustatory abnormality 48.5% |
|
Inomata (Aug 3, 2020) |
Apr 2020 to May 2020 |
15/1,533 |
Ocular symptoms 11.2% (5.5% to 16.9%) |
|
Borsetto (Jul 6,2020) |
Dec 2019 to May 2020 |
18/3,563 |
Alteration of sense of smell or taste 47% (36% to 59%) |
|
Hannum (Jul 6, 2020) |
Jan 2020 to Apr 2020 |
34/19,746 |
Olfactory dysfunction 50.2% (37.7% to 62.6%) |
|
Tong (May 5, 2020) |
Dec 2019 to Apr 2020 |
10/1,627 |
Olfactory dysfunction 52.73% (29.64% to 75.23%)
Gustatory dysfunction 43.93% (20.46% to 68.95%) |
Download Excel Table
Many studies have suggested that early diagnosis, treatment, and prevention of transmission of COVID-19 may be possible by monitoring olfactory/gustatory symptoms as they may appear early in the clinical course of the disease.[12, 24, 34-41] In a meta-analysis of the onset of olfactory/gustatory dysfunction in patients with COVID-19, olfactory/gustatory symptoms preceded or appeared simultaneously with other symptoms in 20% or 28% of cases, respectively.[39] Although the mechanism of olfactory/gustatory dysfunction in COVID-19 is unclear, several hypotheses have been proposed: one that the penetration of the nasal and oral epithelium by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause direct damage to the central nervous system (CNS) cells, as well as olfactory/gustatory receptors[12, 24, 34-41], another that SARS-CoV-2 might cause demyelination of CNS, which stimulates T-cell-mediated autoimmune reactions against CNS antigens,[35] and finally one that epithelial inflammation caused by viral infection may create a barrier to odor chemical.[12, 24, 34-41]
According to a meta-analysis on ocular symptoms of COVID-19 patients, 11.2% of patients with COVID-19 presented ocular symptoms including conjunctivitis, hyperemia, foreign body sensation, chemosis, epiphora, ocular pain, dry eye, floaters, and eyelid dermatitis. Among various ocular symptoms, conjunctivitis (86.3%) was the most common symptom.[42]
3. Conclusion
The symptoms of COVID-19 vary in each organ system. In particular, the prevalence of olfactory/gustatory and psychiatric symptoms was significantly higher than other symptoms. Olfactory/gustatory symptoms appear to be relatively specific symptoms of COVID-19, considering their significantly-high prevalence in COVID-19 compared to other viral or various respiratory diseases. Psychiatric symptoms can be associated with not only the disease itself, but also with the pandemic situation and subsequent quarantine control of the disease. Similar to other viral respiratory diseases, non-specific symptoms such as fever, fatigue, and myalgia were reported commonly. Neurologic symptoms were predominantly non-localized rather than suggestive of focal neurologic deficits. Cardiovascular or chest symptoms were not rare, occurring in about 10% of patients with COVID-19. Although they were not as common as other symptoms, they require a special attention considering its association with severity or mortality. Anorexia, the most common gastrointestinal symptom, may be related to olfactory/gustatory dysfunction in some cases.
Since the symptoms of COVID-19 can vary as described above, it is crucial for clinicians to be informed about them. Estimating the presence of COVID-19 or its contagiousness through presenting symptoms can be of great help for clinicians in a pandemic situation.
Capsule Summary
We summarized meta-analyses of the various extra-pulmonary clinical symptoms of coronavirus disease 2019 (COVID-19) according to organ systems, aiming to provide clinicians with concise information and help administrators set appropriate quarantine policies.