1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic initiated on January 2020.[1] The prolonged COVID-19 pandemic faced a new era following the rapid development of COVID-19 vaccines.[2-4] From December 2020, the US Food and Drug Administration authorized BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Janssen) for emergency use based on the safety and efficacy in clinical trials.[2-4] The randomized clinical trials reported a 95% (95% confidence intervals [95% CI], 90.3 to 96.6) effectiveness of two doses of BNT162b2 vaccine for preventing the COVID-19.[5] For the mRNA-1273 vaccine, its efficacy was estimated to be about 94.1% (95% CI, 89.3 to 96.8).[6] After commencements of COVID-19 vaccination, the subsidence of COVID-19 has been noted since early 2021.[7] However, the emergence of novel variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), such as the delta and omicron, neutralized the effectiveness of COVID-19 vaccination.[8] Moreover, concerns have been made that the vaccine immunity declines over time. To cope with these risks for resurge of COVID-19, the need of a booster dose has been emphasized.[9]
In Korea, the ChAdOx1-S (AstraZeneca; from February 10, 2021), BNT162b2 (from March 5, 2021), Ad26.COV2.S (from April 7, 2021), and mRNA- (from May 21, 2021) were authorized for use by Korea’s Ministry of Food and Drug Safety.[10] More than 80% of Korean have completed the COVID-19 vaccination by January 2022.[10] However, the efficacy of COVID-19 vaccine uptake is still unable to be determined in Korea.
This study investigates the data regarding COVID-19 vaccination in Korea; first whether the cases and proportion of COVID-19 vaccination were analyzed in the entire Korean population, and secondly whether the effects of COVID-19 vaccine regarding the risk reduction for the severe COVID-19 cases were analyzed.
2. Methods
A national official study was conducted with data from the Korea Disease Control and Prevention Agency. The vaccination data for SARS-CoV2 traces back to January 3, 2022. The cases of COVID-19 vaccination of the entire Korean population were collected (total n=51,349,116). All patient records used in this study were anonymized to ensure confidentiality. The study protocol was approved by the Korea Disease Control and Prevention Agency and written informed consent was waived by the ethics commission, owing to the urgent need to collect data.
The distribution of the cases of COVID-19 vaccination was analyzed based on administrative districts. The types of COVID-19 vaccines including ChAdOx1-S, BNT162b2 mRNA-1273, and Ad26.COV2.S vaccines were counted for the first, second, and booster dose of COVID-19 vaccinations. The region of residence was defined as urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) or rural (Gyeonggi, Gangwon, Chungcheongbuk, Chung-cheongnam, Jeollabuk, Jeollanam, Gyeongsangbuk, Gyeongsangnam, and Jeju).[11-13]
The cumulative cases and incidence rates (per 1,000,000 people) of COVID-19 cases were calculated. In addition, the impact of COVID-19 vaccination on the morbidity of COVID-19 was estimated. The severe cases of COVID-19 were counted according to the vaccinated status of unvaccinated, second dosed, and booster dosed COVID-19 vaccinations. The relative risk reduction was analyzed according to the vaccinated status. A 95% confidence interval (95% CI) was calculated through the rate of COVID-19 vaccination. These analyses were performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). A two-sided P value<.05 was considered statistically significant.
3. Results
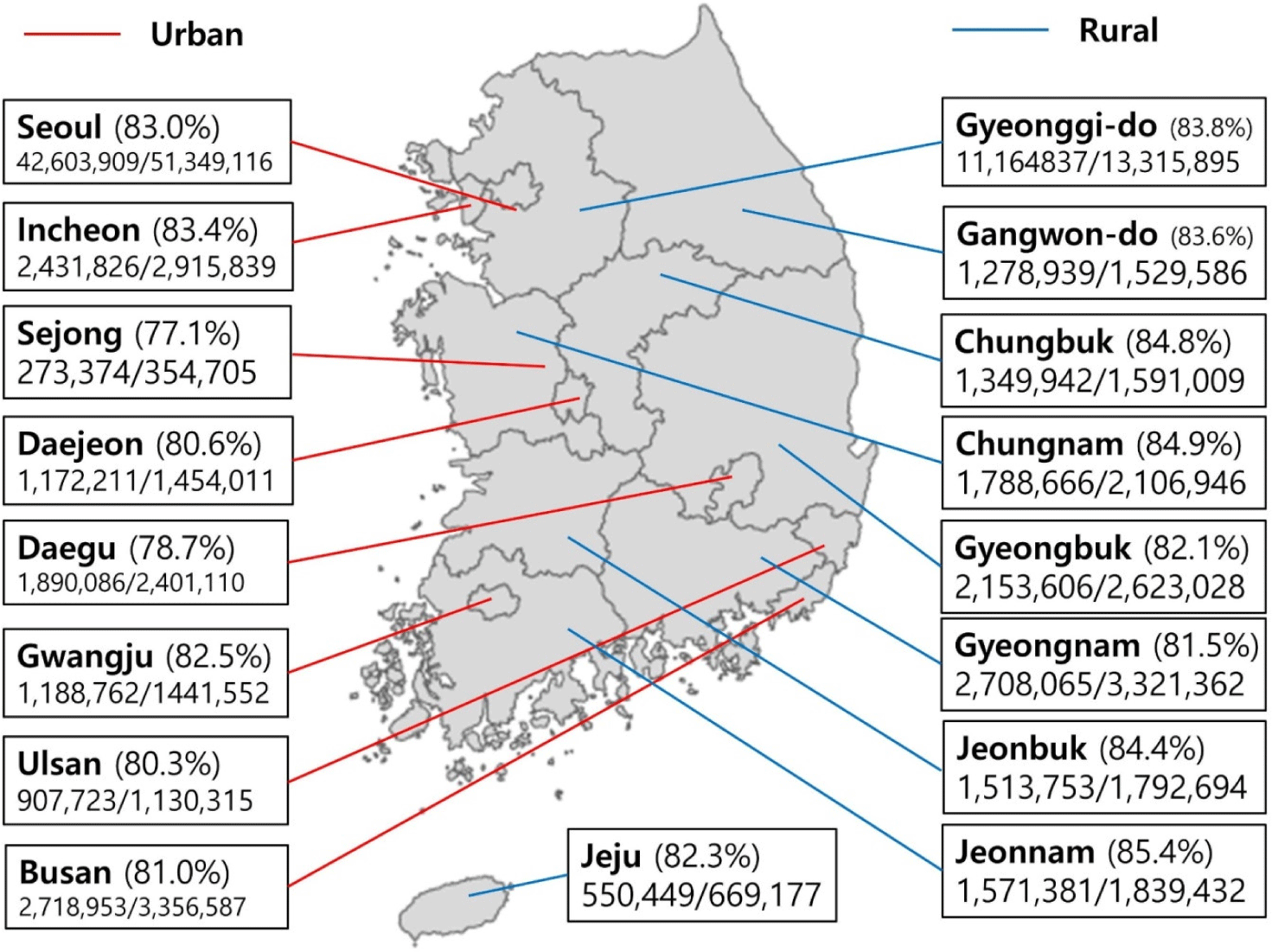
85.30% (43,799,357/51,349,116), 82.22% (42,219,818/51,349,116), and 36.42% (13,709, 545/51,349,116) of the Korean population were vaccinated for each the first, second, and booster doses of COVID-19 vaccines (Table 1). The regional distribution of completed COVID-19 vaccination was from 77.1% to 85.4% (Fig. 1): Seoul 83.0%; Incheon 83.4%; Sejong 77.1%; Daejeon 80.6%; Daegu 78.7%; Gwangju 82.5%; Ulsan 80.3%; Busan 81.0%; Gyeonggi-do 83.8%; Gangwon-do 83.6%; Chungbuk 84.8%; Chungnam 84.9%; Gyeongbuk 82.1%; Gyeongnam 81.5%; Jeonbuk 84.4%; Jeonnam 85.4%; and Jeju 82.3%.
53.42% of the population was vaccinated BNT162b2, 22.56% the mRNA-1273, 19.60% the ChAdOx1s-S, and 4.43% the Ad26.COV2.S for their first dose of COVID-19 vaccinations (Table 2). For the second dose, the rate of BNT162b2 was highest (69.68%), followed by mRNA-1273 (25.44%) and ChAdOx1-S (4.88%). For the booster shot, BNT162b2 vaccination accounted for 52.50%, followed by mRNA-1273 (47.48%) and Ad26.COV2.S (4.95%).
The cumulative number of patients with COVID-19 cases was 642,207, with the incidence rate of 1239 per 1,000,000 people (Table 3). The COVID-19 patients were composed of 51.99% of male and 48.01% of female. The most prevalent groups aged 20 to 69 years old. Among these age groups, the 40 to 49 years old group demonstrated the highest prevalence of COVID-19 (14.62%). On the contrary, the ≥80 years old population showed lowest prevalence of COVID-19 (3.23%).
1.64% of the population which were vaccinated with a second dose and the 0.48% population with booster dose of COVID-19 vaccine were classified as severe COVID-19 cases, which were lower than that of the unvaccinated population (2.25%). The relative risk reduction of severe COVID-19 cases was 0.27 (95% CI, 0.24 to 0.30) for the second dose of COVID-19 vaccine and 0.79 (95% CI, 0.66 to 0.87) for booster dose of COVID-19 vaccine. The relative risk reduction was high in older populations. In the ≥75 years old age group, the relative risk reduction of severe COVID-19 cases was 0.71 (95% CI, 0.69 to 0.73) for the second dose of COVID-19 vaccine and 0.99 (95% CI, 0.99 to 1.00) for the booster dose of COVID-19 vaccine.
4. Discussion
Approximately 82.22% of Koreans were vaccinated a second dose of COVID-19 vaccine and 36.42% of Koreans a booster dose. The COVID-19 vaccination relieved the morbidity of COVID-19 for about 0.27 with a second dose of COVID-19 vaccine and 0.79 with a booster dose. The effects of morbidity reduction were particularly high in old population with COVID-19 vaccination. To our knowledge, this is the first report regarding the connection of COVID-19 vaccination status with the morbidity of COVID-19 in South Korea.
This study demonstrates the reduced risk of severe cases of the COVID-19 vaccinated population. In line with this, several recent studies have reported the preventive effects of COVID-19 vaccine.[14-16] In a case-controlled study in Israel, it was documented that the second dose of BNT162b2 vaccine was effective in reducing the SARS-CoV2 infection, symptomatic cases of COVID-19, hospitalization, and severe cases.[14] The mortality rate from the COVID-19 was reduced to near 72% (95% CI, 19 to 100).[14] In addition to symptomatic COVID-19 cases, the COVID-19 vaccination was also effective in asymptomatic diseases.[15] The observation study in Israel demonstrated lower incidences of both symptomatic and asymptomatic COVID-19 cases in health care workers after the second dose of BNT162b2 vaccine (incidence rate ratio [IRR], 0.03; 95% CI, 0.01 to 0.06 for symptomatic cases and IRR, 0.14; 95% CI, 0.07 to 0.31 for asymptomatic cases).[15] For the ≥70 years old population, both BNT162b2 and ChAdOx1-S vaccine were effective in reducing the hospitalization of COVID-19 cases.[16]
In addition, the booster dose of COVID-19 vaccine documented higher risk reduction than that of the two dose regimen in this study. Increasing evidence supporting the effectiveness of a booster dose of the COVID-19 vaccine is coming to light compared to that of the two dose regimen.[17,18] The booster dose of COVID-19 vaccine was effective for reducing hospitalization (93%; 95% CI, 88 to 97), morbidity (92%; 95% CI, 82 to 97), and mortality (81%; 95% CI, 59 to 97), than those of two doses.[18] In the ≥50 years old population, the hazard ratio for the mortality of COVID-19 was decreased to 0.10 (95% CI, 0.07 to 0.14).[17]
In this study, the effects of COVID-19 vaccination in entire Korean population were first described. The data was provided by the Korea Disease Control and Prevention Agency, in that the accuracy of the data was guaranteed by Korean government.[19, 20] The preventive effects of COVID-19 vaccination were analyzed according to age. However, primarily due to the limited accessibility of the data, the current study could not differentiate the effects of COVID-19 vaccine according to the types of COVID-19 vaccine.[21, 22] In addition, the adverse reactions of COVID-19 vaccinations could not be considered in this study.[22-25] Myocarditis and myopericarditis cases after BNT162b2 COVID-19 vaccinations have been reported in Korea.[23, 24] Moreover, the types of SARS-CoV2 variants were not differentiated for the effects of COVID-19 vaccination in this study. Future studies may solve the current limitations with long-term follow-up data.
5. Conclusion
A maximum of 82.22% of Korean has been vaccinated for COVID-19 by January, 2022. The COVID-19 vaccination was effective for reducing the severity of COVID-19. The booster dose of COVID-19 vaccine presented higher risk reduction effects for severe COVID-19 cases. In addition, the preventive effect of COVID-19 vaccine for severe COVID-19 cases was high in old population.
Our national official results indicated 82.22% of Koreans have successfully been vaccinated two doses and 36.42% were vaccinated a booster dose of the COVID-19 On January 2022.
The COVID-19 vaccination was effective for reducing the severity of the COVID-19 in Korea and the preventive effect of COVID-19 vaccine for severe COVID-19 cases was high in old population.
