1. Introduction
The monkeypox virus (MPXV) is a species of large double-stranded DNA viruses which belongs to the genus Orthopoxvirus in the Poxviridae family.[1] It is the etiological agent of the zoonotic disease monkeypox (MPX), which was first recognized as a human pathogen in the Democratic Republic of the Congo in 1970.[2] After almost 50 years of its discovery, human cases of MPX have been discovered in 11 African countries, which resulted in MPXV being acknowledged as an endemic disease in Africa.[3] However, recent studies have reported that MPX cases were discovered in 107 countries over the course of several months.[4]
2. Global, regional, and national incidence of MPX
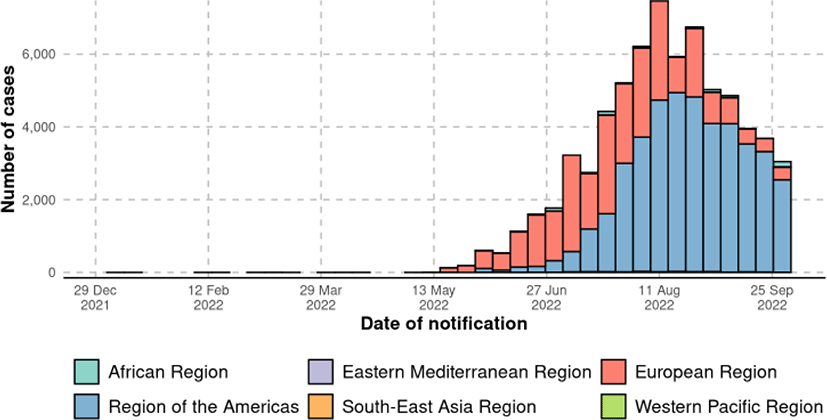
Global, regional, and national estimated incidences of the MPX during the 2022 global outbreak of MPX were analyzed. The cumulative incidence of MPX was calculated based on data from the World Health Organization Dashboard starting since the beginning of the 2022 global outbreak of MPX to October 6th, 2022.[5] Laboratory-confirmed cases were defined based on the World Health Organization guideline.[6] Probable cases were considered when patients had known contact identification and had clinical characteristics such as mucosal lesions. We systematically reviewed the data through World Health Organization dataset.
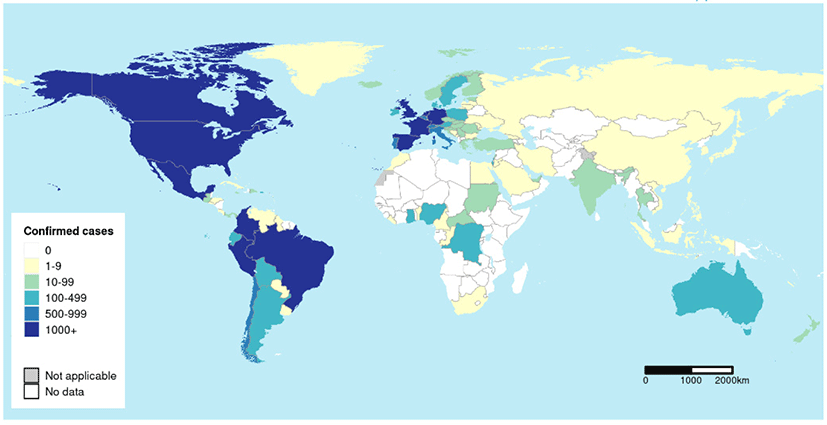
In Fig. 1, the global epidemic curve reported a number of laboratory-confirmed MPX cases up to October 2th, 2022. A total of 71,237 laboratory confirmed MPX cases, 1,097 probable MPX cases, and 26 MPX-related deaths were confirmed globally by October 6th, 2022 (Table 1 and Fig. 2). Among the six World Health Organization regions, Americas demonstrated the highest total laboratory-confirmed MPX cases (45,342 cases), followed by European Region (24,889 cases), African Region (727 cases), Western Pacific Region (189 cases), Eastern Mediterranean Region (67 cases), and South-East Asia Region (23 cases). The nation with the highest cumulative MPX cases was United States of America (26,723 cases), followed by Brazil (8,147 cases), Spain (7209 cases), France (4,043 cases), The United Kingdom (3,654 cases), and Germany (3,640 cases).[7]
3. Are there different monkeypox viruses?
Up to this date, scientists have discovered two clades of MPXV.[8] The West African clade is considered to have developed from western Cameroon to Sierra Leone; in contrast, the Congo Basin clade is known to have occurred from central and southern Cameroon to Congo.[9, 10]
What researchers ascertain from these preliminary genetic data is that the strain of the monkeypox virus detected so far is related to a viral strain predominantly found in West Africa.[11] This strain causes a milder disease and has a lower death rate of about 1% in low income rural populations compared to the one that circulates in Central Africa (which can have a death rate of up to 10%).[11] However, the viruses differ when calculating exactly how much the strain is contributing in the cause of the current outbreaks, and whether the cases developing in different countries are linked to each other remains to be determined by future studies.[12]
4. Why are we observing such an abrupt increase in the number of patients with MPX across different parts of the world?
The reason of such an abrupt increase in the number of patients with MPX around the world may be due to the fact that the MPX derived from a mutation that allows MPX to transmit more readily than it did in the past.[13] In contrast to the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a rapidly evolving RNA virus whose variants have evaded immunity from vaccines and previous infection, MPX is generated by a relatively large DNA virus.[14] It is easier to detect and repair mutations in DNA viruses (i.e., MPXV) than in RNA viruses (i.e., SARS-CoV-2), which indicates that the possibility of the MPXV having a sudden increase in mutations and becoming capable at human-to-human transmission is low.[14, 15] However, the fact that MPX is being detected more than ever in people with no apparent connection to one another proposes the possibility that the virus has been circulating silently.
5. How efficient are the current MPX vaccines and are there other treatment options when infected with MPX?
Currently there are two effective vaccines available against monkeypox. JYNNEOS, (also known as IMVAMUNE, IMVANEX, and MVA-BN) a live viral vaccine produced from the modified vaccinia Ankara-Bavarian Nordic (MVA-BN strain), is an attenuated, non-replicating orthopoxvirus, and is administered subcutaneously in two doses, 28 days apart.[16] ACAM2000 consists of live vaccinia virus, replacing the previous orthopoxvirus vaccine Dryvax, which was withdrawn by the manufacturer.[17] It is given percutaneously by the multiple puncture technique in a single dose using a bifurcated needle.[18] It takes several weeks to develop immunity after being vaccinated. Although most patients with MPX have a mild and self-limited disease, treatment options including antivirals (i.e., tecovirimat, brincidofovir[19, 20], and cidofovir[21, 22]) and vaccinia immune globulin intravenous are available for seriously ill or immunocompromized patients.[18] The other vaccine, the Aventis Pasteur Smallpox Vaccine, can be utilized for MPX under an investigational new drug protocol.[18] The U.S. Centers for Disease Control recommends that the first dose of vaccine be administered within 4 days of exposure to prevent disease. If given 4-14 days after the exposure date, vaccination might contribute in decreasing the signs and/or symptoms of the disease but not prevent the disease onset.[23] However, mass vaccination is not advocated at this time.
Vaccinia immune globulin is a hyperimmune globulin licensed by the U.S. Food and Drug Administration for treatment of certain complications of vaccinia vaccination.[24] Despite that it is a possible treatment option, data regarding the efficacy of vaccinia immune globulin against MPX and smallpox is generally incomplete; therefore, the administration of vaccinia immune globulin for MPX or smallpox has not been adequately assessed in humans. Due to the fact vaccination with vaccinia virus vaccine is contraindicated in subjects with severe immunodeficiency in T-cell function, such individuals with exposure history may alternatively be administered vaccinia immune globulin.[25]
6. Conclusion
Most patients with MPX have a mild, self-limiting disease course with no medical treatment or supportive care; however, the prognosis for MPX may be different in accordance with initial health status (i.e., immunocompromised patients), previous vaccination status, and comorbidities. Taken together, our results highlight that up to this date, a case-by-case strategy can be seen as the most reasonable therapeutic option.
